EP0440472B1 - High bulking resilient fibers through cross linking of wood pulp fibers with polycarboxylic acids - Google Patents
High bulking resilient fibers through cross linking of wood pulp fibers with polycarboxylic acids Download PDFInfo
- Publication number
- EP0440472B1 EP0440472B1 EP91300760A EP91300760A EP0440472B1 EP 0440472 B1 EP0440472 B1 EP 0440472B1 EP 91300760 A EP91300760 A EP 91300760A EP 91300760 A EP91300760 A EP 91300760A EP 0440472 B1 EP0440472 B1 EP 0440472B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fibers
- wood pulp
- crosslinked
- drying
- bulking
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C9/00—After-treatment of cellulose pulp, e.g. of wood pulp, or cotton linters ; Treatment of dilute or dewatered pulp or process improvement taking place after obtaining the raw cellulosic material and not provided for elsewhere
- D21C9/001—Modification of pulp properties
- D21C9/002—Modification of pulp properties by chemical means; preparation of dewatered pulp, e.g. in sheet or bulk form, containing special additives
- D21C9/005—Modification of pulp properties by chemical means; preparation of dewatered pulp, e.g. in sheet or bulk form, containing special additives organic compounds
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H11/00—Pulp or paper, comprising cellulose or lignocellulose fibres of natural origin only
- D21H11/16—Pulp or paper, comprising cellulose or lignocellulose fibres of natural origin only modified by a particular after-treatment
- D21H11/20—Chemically or biochemically modified fibres
Definitions
- the present invention relates generally to fibers exhibiting improved resilient bulking and absorbent properties and paper products comprising said fibers. More particularly, this invention relates to an improved method of preparing resilient bulking fibers by crosslinking wood pulp fibers with polycarboxylic acids.
- resilient bulking fibers are useful for the preparation of bulkier and more absorbent paper structures. Such paper structures are useful for the manufacture of products such as handsheets, towels, tissues, filters, paperboard, diapers, sanitary napkins, hospital dressings and the like.
- One method for obtaining resilient bulking fibers is by crosslinking cellulose fibers by treatment with a chemical compound.
- US-A-3,819,470 discloses modified cellulosic fibers characterized by reduced swellability and a reduced capability of natural fiber-to-fiber bonding when compared to unmodified cellulosic fibers and having a substantive polymeric compound reacted with and attached to the fibers.
- US-A-4,431,481 discloses modified cellulosic fibers produced by treating the fibers with copolymers of maleamic acid.
- Other known techniques include treatment of fibers with cationic urea formaldehyde resins, (US-A-3,756,913), methylol ureas and melamines (US-A-3,440,135), formaldehyde (US-A-3,224,926), with the condensation product of acrolein and formaldehyde, (US-A-3,183,054), bis-acrylamides (EP-A-0,213,415), and treatment with glyoxal or glutaric dialdehyde (WO-A-88104704, US-A-4,822,453 and US-A-4,853,086).
- US-A-4822453 proposes the use of an organic acid such as citric acid in combination with zinc nitrate as a catalyst for the crosslinking action.
- crosslinking methods of the prior art tend to suffer from the disadvantages of toxicity, high cost, or poor effectiveness. Of these, toxicity is especially disadvantageous in view of the mounting concerns over the environment and safety of the workers. Because of these concerns, most currently available bulking fibers and the methods for making them are not commercially acceptable or will be challenged.
- crosslinkers such as epichlorohydrin, divinylsulfone, bisacrylamides, formaldehyde, and formaldehyde-based reagents such as 4,5-dihydroxy-1,2-dimethylol-ethylene urea (common textile finish) present serious hazards to workers and consumers.
- Formaldehyde-free reagents such as 4,5-dihydroxy-1, 2-dimethyl-ethylene urea, while safer, are very expensive.
- Other formaldehyde-free reagents such as glyoxal, glutaric dialdehyde, and various resins, while generally considered non-hazardous and reasonably priced, are less effective at producing bulking resilient fibers.
- treatment of cellulosic fibers with maleamic copolymers or other resins results in fibers having equivalent bulk to fibers without chemical treatment that were heated to the same elevated temperatures as utilized with the resin treatment.
- Nit formation is particularly prevalent when faster reacting agents, such as aldehydic compounds, or when polymeric agents are used.
- Practitioners of the art usually employ debonding agents, mechanical defibration such as hammermilling, and screening to reduce the nit and knot contents of treated fibers. Such measures tend to be costly and can be deleterious to fiber and paper quality.
- the present invention overcomes the problems and disadvantages of the prior art directed to papermaking by providing high bulking resilient fibers with little or no nits or knots obtained through crosslinking of wood pulp fibers with polycarboxylic acids such as citric acid.
- Another object of the present invention is to increase the anionicity of the fibers such that the fibers are more receptive to specific additives and are themselves more conducive to making acceptable paper substrates.
- a resilient bulking fiber comprising individualized crosslinked wood pulp cellulosic fibers having intra-fiber chemical bonds characterised in that the intra-fiber crosslink bonds derive from a polycarboxylic acid, and the degree of crosslinking is at least that sufficient to induce in said individualized fibers at least one of the following, namely twisting, curling and resilient bulking tendency.
- crosslinking is intra-fiber; that is the crosslink bonds are primarily between cellulose molecules of a single fiber. This is in contrast to inter-fiber cross-linking where the bonds are formed between cellulose molecules of different fibers.
- the resulting dry bulking fibers can be incorporated into products through conventional papermaking techniques. These fibers resist relaxation during papermaking, retaining their bulking behaviour throughout the papermaking process.
- the invention further provides the use of polycarboxylic acid as the cross-linking agent to induce twisting and curling in individualized wood pulp cellulosic fibers by the formation of intra-fiber crosslink bonds.
- an absorbent paper product comprising cross-linked wood pulp cellulose fibers in accordance with the invention to provide improved bulking and absorbent properties.
- the crosslinked fibers may contain both intra-fiber and interfiber bonds.
- the paper product may also contain non-crosslinked fibers which may be wood fibers and which may comprise the majority of the product on a weight basis. Examples of wood fibers are pre-dried and never dried Scandinavian bleached spruce kraft, Southern pine bleached kraft, secondary fibers, Southern and Northern softwood krafts and never dried Northern softwood bleached kraft.
- the paper products may be, for example, handsheets, towels, tissues, filters, paperboard, diapers, sanitary napkins and hospital dressings.
- Fig. 1 graphically depicts the Attenuated Total Reflectance (ATR) of CAFC fibers (cf Example 4).
- Fig. 2 graphically depicts the ATR spectrum of TC fibers (cf Example 2).
- Fig. 3 graphically depicts the ATR spectrum of CA fibers (cf Example 6).
- Fig. 4 is a microphotograph of fibers that were oven dried and cured without citric acid.
- Fig. 5 is a microphotograph of fibers that were oven dried and cured with citric acid.
- resilient bulking fibers and a method for their preparation by crosslinking individualized wood pulp cellulose fibers with polycarboxylic acids.
- individualized crosslinked fibers refers to cellulosic fibers that have primarily intrafiber chemical crosslink bonds. That is, the crosslink bonds are primarily between cellulose molecules of a single fiber, rather than between cellulose molecules of separate fibers.
- the cellulose fibers are treated with an aqueous solution comprising a polycarboxylic acid and, if desired, an additional agent such as sodium hydroxide or other caustic agent or a coreactant/accelerator. It is preferable to select the coreactant/accelerator from the class of inorganic phosphorus compounds. It is more preferable to select the coreactant/accelerator from the group consisting of phosphates, phosphites, hypophosphites, pyrophosphates and metaphosphates. It is most preferable to use an inorganic phosphorus compound such as monosodium phosphate.
- Dry lap or never dried wood pulp fibers can be used, although it is preferable to use never dried fibers. It is our experience that starting with the never-dried fiber results in maximum bulking levels after crosslinking regardless of the type of cellulose crosslinker used. Not wishing to be bound by any theory, it is believed that never-dried fibers allow for homogeneous distribution of crosslinking chemical in the cell wall, remain in a more individualized state during the crosslinking process, and more readily adopt twisted and curled configurations than do predried fibers.
- wood pulp fibers may be used, although it is preferable to use chemical thermal mechanical pulps, Southern and Northern softwood bleached kraft pulps, and secondary fibers.
- individualized wood pulp cellulosic fibers are crosslinked by a polycarboxylic acid.
- the degree of crosslinking is at least that sufficient to induce twisting and curling and/or resilient bulking tendency in said individualized fibers.
- the upper limit would be reached when the degree of crosslinking renders the fibers unfit for the intended use.
- Individualized crosslinked fibers according to this invention thus include those crosslinked by from less than 1 mole % to more than 25 mole %, calculated on a cellulosic anhydroglucose molar basis, of a polycarboxylic acid crosslinking agent, although from 1 to 25 mole % is preferred.
- any polycarboxylic acid known to crosslink cellulose may be used to crosslink the fibers according to the present invention.
- Preferred polycarboxylic acids include citric acid, propane tricarboxylic acid, maleic acid, butanetetracarboxylic acid, cyclopentanetetracarboxylic acid and benzene tetracarboxylic acid. It is also contemplated to use polycarboxylic acid precursors and derivatives that will produce the polycarboxylic acid under the reaction conditions utilized to crosslink the fibers.
- the most preferred polycarboxylic acid is citric acid because it is an inexpensive, nontoxic, environmentally safe, readily available, naturally occurring polycarboxylic acid.
- the polycarboxylic acid may be present in any concentration in the aqueous solution to allow for a sufficient number of crosslinks. It is advantageous to use in the range of a 3-10% aqueous solution of polycarboxylic acid, with about a 5% aqueous solution being most preferred.
- a caustic agent may be used, if desired, including sodium hydroxide.
- the fibers may be dewatered by conventional papermaking techniques, for example, through the use of a screw press.
- the dewatering is done to any consistency, although higher consistencies are desirable for economical drying.
- the fibers are dewatered to a consistency of at least 30%.
- it is important to minimize compression forces experienced by the fibers prior to crosslinking and particularly during dewatering.
- the dewatered fibers may be dried by any method that allows individualization of fibers (i.e., minimizes nits, knots, fisheyes, etc.).
- the fibers may be azeotropically dried in a solvent, preferably toluene.
- the filtered (i.e. dewatered) fibers may be fluff dried using a hot gas such as air or superheated steam.
- the fibers After the fibers have been dried to an individualized state, they are then cured by conventionally known means to bring about the crosslinking reaction.
- the fibers may be cured by heating them at a temperature in the range of from 150°C to 180°C for in the range of about one-half of a minute to about ten minutes.
- Drying and curing can be accomplished either separately or concurrently in either batch or continuous operations.
- Drying and curing of the treated fibers can be achieved by any means that allows heating of the fibers to elevated temperatures, for example, ovens, or heating in hot gas streams such as air, steam, superheated steam, or inert gases such as argon or nitrogen. It is preferred to use reducing atmospheres during drying and curing, such as is achievable with systems like superheated steam or inert gases like nitrogen and argon, to minimize charring, darkening, and degradation of the fibers.
- the cured fibers thus prepared can then be dispersed for use.
- the dispersion step involves contacting the cured fibers with water at an elevated temperature.
- These bulking fibers may then be used -- alone or in blends -- to prepare products that exhibit improved bulking and absorbent properties.
- the improvement in absorbency relates both to faster rate of absorbency and to increased fluid-holding capacity.
- the amounts of crosslinked fibers used to prepare the products are readily determinable by those skilled in the art. For instance, filtration and absorbent product applications will often be made 100% from the fibers of the present invention.
- towel and tissue paper products may be made by blending fibers according to the present invention with a majority of conventional wood pulp fibers. In such applications, it may be preferable to use crosslinked fibers in an amount of 25% or less by weight of the paper product.
- NSWK Northern bleached softwood kraft fibers
- Example 1 was repeated without citric acid to produce fibers hereafter referred to as "TC".
- Example 1 was repeated except that no sodium hydroxide was added to the citric acid solution , the fibers were fluff dried with hot air in lieu of azeotrope drying in toluene, and curing was done at 180°C for 2.8 minutes.
- the resultant fibers are hereafter referred to as "CAFC".
- Example 4 was repeated without citric acid to generate fibers hereafter referred to as "FC".
- Example 4 was repeated without the oven curing step to generate fibers hereafter referred to as "CA".
- Example 4 was repeated without citric acid and without the oven curing step to generate fibers hereafter referred to as "FD".
- the citric acid crosslinking reaction rendered the NSWK fiber more anionic. This was readily apparent by treating the crosslinked fibers with methylene blue. A deep blue color was retained in the crosslinked fibers, whereas little dye was taken up by the untreated NSWK fibers.
- the total charge of citric acid crosslinked fibers, made according to Example 4 was 76 meq/100 g.
- the total charge of untreated fibers was 4 meq/100 g.
- This anionicity is a further advantage of the fibers of the present invention over those prepared according to the past art, as the polycarboxylic acid crosslinked fibers should be more receptive to cationic additives important to papermaking. For example, the strength of sheets made from the crosslinked fibers should be recoverable without compromising the bulk enhancement by incorporation of a cationic strength resin.
- the polycarboxylic acid crosslinking reaction did not appear to damage the NSWK fibers. Thus, the average fiber length was not changed by the crosslinking reaction. Furthermore, the integrity of the fibers was unchanged by the crosslinking reaction as evidenced by microscopic examination (compare Figures 4 and 5). There was some brightness reduction due to the crosslinking reaction (see Table 1).
- Example 4 Partial neutralization of the citric acid prior to fiber treatment is not necessary (See Example 4) for the successful preparation of high bulking resilient fibers as described above.
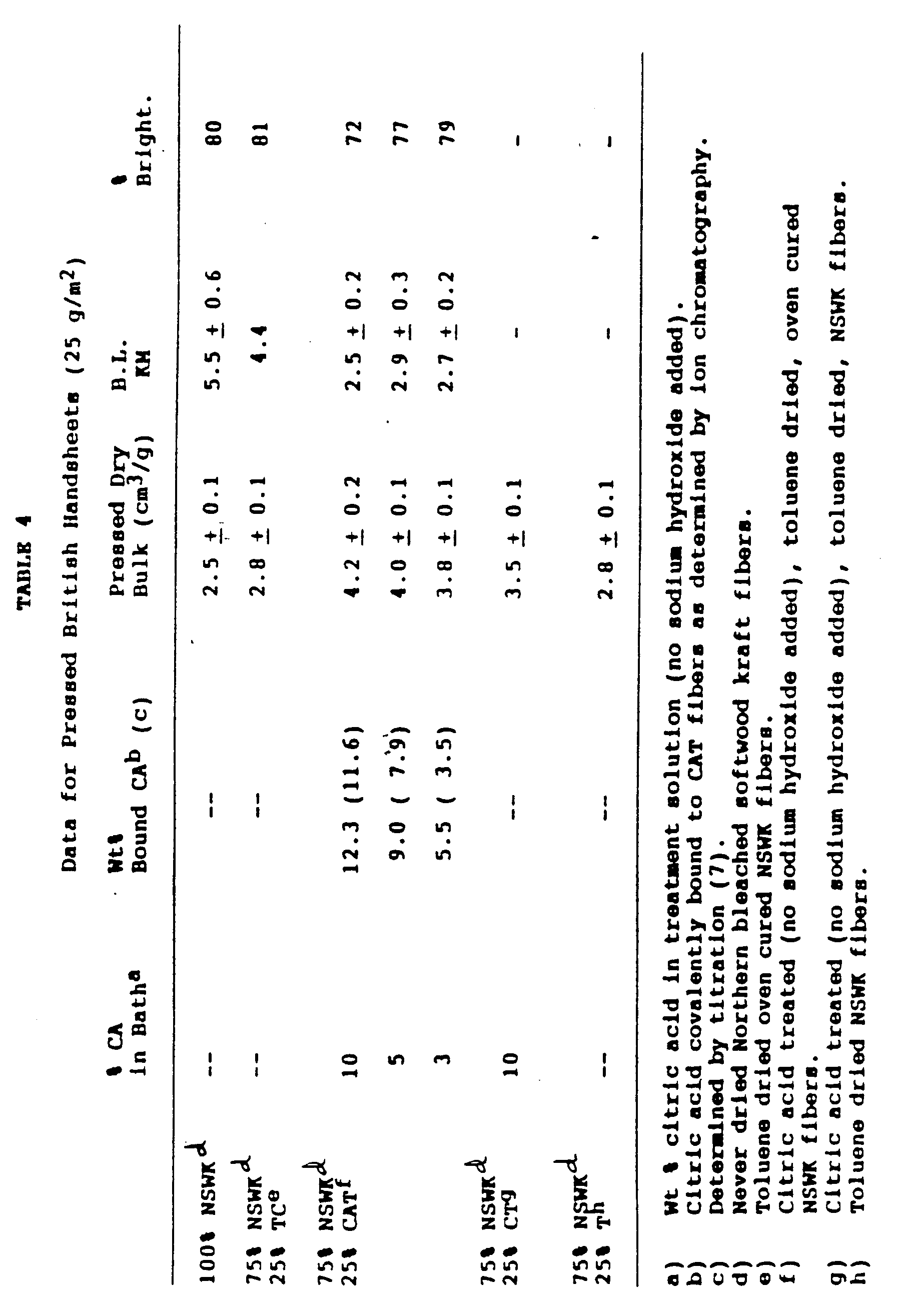
- Examples 1-3 were repeated without the use of sodium hydroxide in the preparation of the treatment solution, and the resultant fibers (i.e. CAT fibers) had equivalent performance to that of the CATC fibers (compare data in Table 4 with that in Table 1). Since the citric acid treated fibers were at 25% consistency prior to drying, 39% of available citric acid (i.e. that acid contained in the dry fiber prior to curing) had reacted with the NSWK fibers to produce the CAT fibers described in this example.
- Example 10 was repeated except a treatment solution containing only 5 wt% citric acid was used.
- Table 4 comparable bulking performance is observed with the resultant CAT fibers relative to those prepared with solutions having twice the level of citric acid. Furthermore, there is a marked improvement in brightness accompanying the reduction of citric acid in the treatment bath. It should also be noted that 53% of the available citric acid had reacted with the NSWK fibers to produce the CAT fibers described in this example.
- Example 10 was repeated except a 3 wt% aqueous solution of citric acid was used for the treatment. As can be seen in Table 4, there was a slight reduction in the bulking ability realized with the CAT fiber prepared under these conditions. Nevertheless, a 2% bulk enhancement is predicted for every 1% incorporation of these fibers in a NSWK furnish. Furthermore, essentially no reduction in brightness was observed with these fibers relative to the control. As was the case with the CAT fibers prepared according to Example 10, about 39% of the available citric acid had reacted with the NSWK fibers to produce the CAT fibers described in this example.
- the percent bound citric acid levels as determined by titration (7) are consistently lower than those determined by ion chromatography.
- the latter method is considered to be more reliable as it is not predicated on an assumption of the number of active equivalents of carboxyl functionality during base hydrolysis.
- the citric acid crosslinking treatment is effective at producing bulk and resiliency enhancement in a wide variety of wood pulps. Different wood pulps were treated according to Example 13, unless otherwise stated, and made into pressed 65 g/m2 handsheets. The bulk data is provided in Table 6.
Abstract
Description
- The present invention relates generally to fibers exhibiting improved resilient bulking and absorbent properties and paper products comprising said fibers. More particularly, this invention relates to an improved method of preparing resilient bulking fibers by crosslinking wood pulp fibers with polycarboxylic acids.
- It is known in the art that resilient bulking fibers are useful for the preparation of bulkier and more absorbent paper structures. Such paper structures are useful for the manufacture of products such as handsheets, towels, tissues, filters, paperboard, diapers, sanitary napkins, hospital dressings and the like. One method for obtaining resilient bulking fibers is by crosslinking cellulose fibers by treatment with a chemical compound. US-A-3,819,470 discloses modified cellulosic fibers characterized by reduced swellability and a reduced capability of natural fiber-to-fiber bonding when compared to unmodified cellulosic fibers and having a substantive polymeric compound reacted with and attached to the fibers. US-A-4,431,481 (equivalent to EP-A-0090588) discloses modified cellulosic fibers produced by treating the fibers with copolymers of maleamic acid. Other known techniques include treatment of fibers with cationic urea formaldehyde resins, (US-A-3,756,913), methylol ureas and melamines (US-A-3,440,135), formaldehyde (US-A-3,224,926), with the condensation product of acrolein and formaldehyde, (US-A-3,183,054), bis-acrylamides (EP-A-0,213,415), and treatment with glyoxal or glutaric dialdehyde (WO-A-88104704, US-A-4,822,453 and US-A-4,853,086). US-A-4822453 proposes the use of an organic acid such as citric acid in combination with zinc nitrate as a catalyst for the crosslinking action.
- The crosslinking methods of the prior art, however, tend to suffer from the disadvantages of toxicity, high cost, or poor effectiveness. Of these, toxicity is especially disadvantageous in view of the mounting concerns over the environment and safety of the workers. Because of these concerns, most currently available bulking fibers and the methods for making them are not commercially acceptable or will be challenged.
- Thus, crosslinkers such as epichlorohydrin, divinylsulfone, bisacrylamides, formaldehyde, and formaldehyde-based reagents such as 4,5-dihydroxy-1,2-dimethylol-ethylene urea (common textile finish) present serious hazards to workers and consumers. Formaldehyde-free reagents such as 4,5-dihydroxy-1, 2-dimethyl-ethylene urea, while safer, are very expensive. Other formaldehyde-free reagents such as glyoxal, glutaric dialdehyde, and various resins, while generally considered non-hazardous and reasonably priced, are less effective at producing bulking resilient fibers. For example, treatment of cellulosic fibers with maleamic copolymers or other resins, as taught in US-A-4,431,481, results in fibers having equivalent bulk to fibers without chemical treatment that were heated to the same elevated temperatures as utilized with the resin treatment.
- The formation of nits and knots is a common problem in the preparation of bulking resilient fibers through chemical crosslinking. Nit formation is particularly prevalent when faster reacting agents, such as aldehydic compounds, or when polymeric agents are used. Practitioners of the art usually employ debonding agents, mechanical defibration such as hammermilling, and screening to reduce the nit and knot contents of treated fibers. Such measures tend to be costly and can be deleterious to fiber and paper quality.
- The prior art does not disclose the use of polycarboxylic acids as crosslinkers or coreactants with other crosslinking systems for the production of bulking absorbent fibers, although the textile industry has demonstrated the use of polycarboxylic acids as crosslinkers or coreactants with other crosslinkers for the enhancement of wrinkle-resistance and durable-press properties in cotton fabrics (US-A-3,526,048 and US-A-4,820,307, and Text. Res. J. (1967), 37, 933 and (1972), 42, 274). Also cellulosic fibers and powders have been crosslinked with citric acid to produce ion exchange materials (US-A-2,759,787).
- The present invention overcomes the problems and disadvantages of the prior art directed to papermaking by providing high bulking resilient fibers with little or no nits or knots obtained through crosslinking of wood pulp fibers with polycarboxylic acids such as citric acid.
- It is an object of the present invention to provide such resilient bulking fibers in a manner which will minimize the cost and increase the effectiveness of the fibers produced.
- It is an additional object of the present invention to minimize the hazards to workers and the environment during preparation of these fibers.
- Another object of the present invention is to increase the anionicity of the fibers such that the fibers are more receptive to specific additives and are themselves more conducive to making acceptable paper substrates.
- Additional objects and advantages of the invention will be set forth in the description which follows, and in part will be apparent from the description, or may be learned by practice of the invention. The objects and advantages of the invention may be realized and obtained by means of the instrumentalities and combinations particularly pointed out in the appended claims.
- To achieve the foregoing objects, and in accordance with the purposes of the invention as embodied and broadly described herein, there is provided a resilient bulking fiber comprising individualized crosslinked wood pulp cellulosic fibers having intra-fiber chemical bonds characterised in that the intra-fiber crosslink bonds derive from a polycarboxylic acid, and the degree of crosslinking is at least that sufficient to induce in said individualized fibers at least one of the following, namely twisting, curling and resilient bulking tendency.
- There is also provided a method for preparing resilient bulking fibers by:
- (a) contacting wood pulp cellulosic fibers with a crosslinking agent;
- (b) individualizing the cellulosic fibers; and
- (c) curing the individualized cellulosic fibers to form intra-fiber cross-link bonds derived from said crosslinking agent between cellulose molecules within individual fibers of the cellulosic fibers, characterised in that a polycarboxylic acid is employed as the crosslinking agent.
- Individualizing the treated fibers prior to heating them to effect crosslinking ensures that the crosslinking is intra-fiber; that is the crosslink bonds are primarily between cellulose molecules of a single fiber. This is in contrast to inter-fiber cross-linking where the bonds are formed between cellulose molecules of different fibers. The resulting dry bulking fibers can be incorporated into products through conventional papermaking techniques. These fibers resist relaxation during papermaking, retaining their bulking behaviour throughout the papermaking process.
- The invention further provides the use of polycarboxylic acid as the cross-linking agent to induce twisting and curling in individualized wood pulp cellulosic fibers by the formation of intra-fiber crosslink bonds.
- Also provided is an absorbent paper product comprising cross-linked wood pulp cellulose fibers in accordance with the invention to provide improved bulking and absorbent properties. The crosslinked fibers may contain both intra-fiber and interfiber bonds. The paper product may also contain non-crosslinked fibers which may be wood fibers and which may comprise the majority of the product on a weight basis. Examples of wood fibers are pre-dried and never dried Scandinavian bleached spruce kraft, Southern pine bleached kraft, secondary fibers, Southern and Northern softwood krafts and never dried Northern softwood bleached kraft. The paper products may be, for example, handsheets, towels, tissues, filters, paperboard, diapers, sanitary napkins and hospital dressings.
- Fig. 1 graphically depicts the Attenuated Total Reflectance (ATR) of CAFC fibers (cf Example 4).
- Fig. 2 graphically depicts the ATR spectrum of TC fibers (cf Example 2).
- Fig. 3 graphically depicts the ATR spectrum of CA fibers (cf Example 6).
- Fig. 4 is a microphotograph of fibers that were oven dried and cured without citric acid.
- Fig. 5 is a microphotograph of fibers that were oven dried and cured with citric acid.
- Reference will now be made in detail to the present preferred embodiment of the invention. In accordance with the present invention there is provided resilient bulking fibers and a method for their preparation by crosslinking individualized wood pulp cellulose fibers with polycarboxylic acids. The terminology "individualized crosslinked fibers" as used herein, refers to cellulosic fibers that have primarily intrafiber chemical crosslink bonds. That is, the crosslink bonds are primarily between cellulose molecules of a single fiber, rather than between cellulose molecules of separate fibers.
- The cellulose fibers are treated with an aqueous solution comprising a polycarboxylic acid and, if desired, an additional agent such as sodium hydroxide or other caustic agent or a coreactant/accelerator. It is preferable to select the coreactant/accelerator from the class of inorganic phosphorus compounds. It is more preferable to select the coreactant/accelerator from the group consisting of phosphates, phosphites, hypophosphites, pyrophosphates and metaphosphates. It is most preferable to use an inorganic phosphorus compound such as monosodium phosphate.
- Dry lap or never dried wood pulp fibers can be used, although it is preferable to use never dried fibers. It is our experience that starting with the never-dried fiber results in maximum bulking levels after crosslinking regardless of the type of cellulose crosslinker used. Not wishing to be bound by any theory, it is believed that never-dried fibers allow for homogeneous distribution of crosslinking chemical in the cell wall, remain in a more individualized state during the crosslinking process, and more readily adopt twisted and curled configurations than do predried fibers.
- Any wood pulp fibers may be used, although it is preferable to use chemical thermal mechanical pulps, Southern and Northern softwood bleached kraft pulps, and secondary fibers.
- According to the present invention, individualized wood pulp cellulosic fibers are crosslinked by a polycarboxylic acid. The degree of crosslinking is at least that sufficient to induce twisting and curling and/or resilient bulking tendency in said individualized fibers. The upper limit would be reached when the degree of crosslinking renders the fibers unfit for the intended use.
- Individualized crosslinked fibers according to this invention thus include those crosslinked by from less than 1 mole % to more than 25 mole %, calculated on a cellulosic anhydroglucose molar basis, of a polycarboxylic acid crosslinking agent, although from 1 to 25 mole % is preferred.
- Any polycarboxylic acid known to crosslink cellulose may be used to crosslink the fibers according to the present invention. Preferred polycarboxylic acids include citric acid, propane tricarboxylic acid, maleic acid, butanetetracarboxylic acid, cyclopentanetetracarboxylic acid and benzene tetracarboxylic acid. It is also contemplated to use polycarboxylic acid precursors and derivatives that will produce the polycarboxylic acid under the reaction conditions utilized to crosslink the fibers. The most preferred polycarboxylic acid is citric acid because it is an inexpensive, nontoxic, environmentally safe, readily available, naturally occurring polycarboxylic acid.
- The polycarboxylic acid may be present in any concentration in the aqueous solution to allow for a sufficient number of crosslinks. It is advantageous to use in the range of a 3-10% aqueous solution of polycarboxylic acid, with about a 5% aqueous solution being most preferred.
- A caustic agent may be used, if desired, including sodium hydroxide.
- After the fibers are treated with the aqueous solution, the fibers may be dewatered by conventional papermaking techniques, for example, through the use of a screw press. The dewatering is done to any consistency, although higher consistencies are desirable for economical drying. Preferably, the fibers are dewatered to a consistency of at least 30%. In order to maximize the bulking and resilient characteristics of the crosslinked fibers, it is important to minimize compression forces experienced by the fibers prior to crosslinking and particularly during dewatering.
- The dewatered fibers may be dried by any method that allows individualization of fibers (i.e., minimizes nits, knots, fisheyes, etc.). For example the fibers may be azeotropically dried in a solvent, preferably toluene. Alternatively, the filtered (i.e. dewatered) fibers may be fluff dried using a hot gas such as air or superheated steam.
- After the fibers have been dried to an individualized state, they are then cured by conventionally known means to bring about the crosslinking reaction. For example, the fibers may be cured by heating them at a temperature in the range of from 150°C to 180°C for in the range of about one-half of a minute to about ten minutes.
- Drying and curing can be accomplished either separately or concurrently in either batch or continuous operations.
- In order to maximize the bulking and resilient characteristics of the fibers prepared according to the present invention it is desirable to conduct drying at a lower temperature than that used for curing.
- Drying and curing of the treated fibers can be achieved by any means that allows heating of the fibers to elevated temperatures, for example, ovens, or heating in hot gas streams such as air, steam, superheated steam, or inert gases such as argon or nitrogen. It is preferred to use reducing atmospheres during drying and curing, such as is achievable with systems like superheated steam or inert gases like nitrogen and argon, to minimize charring, darkening, and degradation of the fibers.
- The cured fibers thus prepared can then be dispersed for use. Preferably, the dispersion step involves contacting the cured fibers with water at an elevated temperature.
- These bulking fibers may then be used -- alone or in blends -- to prepare products that exhibit improved bulking and absorbent properties. The improvement in absorbency relates both to faster rate of absorbency and to increased fluid-holding capacity. The amounts of crosslinked fibers used to prepare the products are readily determinable by those skilled in the art. For instance, filtration and absorbent product applications will often be made 100% from the fibers of the present invention. On the other hand, towel and tissue paper products may be made by blending fibers according to the present invention with a majority of conventional wood pulp fibers. In such applications, it may be preferable to use crosslinked fibers in an amount of 25% or less by weight of the paper product.
- Additional advantages and modifications will readily occur to those skilled in the art. The invention in its broader aspects is, therefore, not limited to the specific details and illustrative examples shown and described. Accordingly, departures may be made from such details without departing from the spirit or scope of the general inventive concept as defined by the appended claims and their equivalents.
- The following examples further illustrate preferred embodiments of the present invention. The examples should in no way be considered limiting, but are merely illustrative of the various features of the present invention.
- Never dried Northern bleached softwood kraft fibers (NSWK) were dispersed in a 10% aqueous solution of citric acid, to which 0.03 equivalents of sodium hydroxide (based on equivalents citric acid) had been added. The resultant fibers were filtered to approximately 30% consistency, azeotropically dried in toluene, filtered, and heated in an oven at 160°C for 10 min. The cured fibers were then disintegrated in 100°C water for 30 min. (the water temperature drops to 45°C during this time). The resultant fibers are hereafter referred to as "CATC".
- Example 1 was repeated without citric acid to produce fibers hereafter referred to as "TC".
- The fibers described in Examples 1 and 2 were made into pressed British handsheets according to standard methods using the furnish compositions described in Table 1. As can be seen from the data provided in Table 1, sheets made with the furnish containing the CATC fibers had the highest bulk after pressing. Thus, for every 1% incorporation of CATC fibers in a furnish containing NSWK fibers, a 2.5% increase in dry sheet bulk was seen after pressing.
- Example 1 was repeated except that no sodium hydroxide was added to the citric acid solution , the fibers were fluff dried with hot air in lieu of azeotrope drying in toluene, and curing was done at 180°C for 2.8 minutes. The resultant fibers are hereafter referred to as "CAFC".
- Example 4 was repeated without citric acid to generate fibers hereafter referred to as "FC".
- Example 4 was repeated without the oven curing step to generate fibers hereafter referred to as "CA".
- Example 4 was repeated without citric acid and without the oven curing step to generate fibers hereafter referred to as "FD".
- The fibers obtained in Examples 4-7 were used to prepare British handsheets as described in Example 3. The pressed bulk data for the resultant sheets are provided in Table 2.
- The crosslinking presumably occurs by the formation of diester bonds between cellulose chains. The existence of ester linkages in the CATC and CAFC fibers is clearly evident from the band at 1728 cm⁻¹ obtained by IR spectroscopy (for example see Figure 1). Such ester linkages are absent in the untreated or uncured fibers (for examples see Figures 2 and 3). The percent covalently bound citric acid was measured in the CAFC fibers by titration according to the method described in Text. Res. J. (1967), 37:933 and found to be 7 wt% (based on weight of oven dried fiber). This means that 23% of the available citric acid had actually reacted with the fiber.
- The citric acid crosslinking reaction appeared to impart additional kink and curl to the fibers that were otherwise not achieved by the heat treatments alone. This suggestion was supported by comparison of microphotographs of fibers that were oven dried and cured without citric acid (Figure 4) with microphotographs of fibers that were oven dried and cured with citric acid (Figure 5).
- The citric acid crosslinking reaction rendered the NSWK fiber more anionic. This was readily apparent by treating the crosslinked fibers with methylene blue. A deep blue color was retained in the crosslinked fibers, whereas little dye was taken up by the untreated NSWK fibers. The total charge of citric acid crosslinked fibers, made according to Example 4, was 76 meq/100 g. The total charge of untreated fibers was 4 meq/100 g. This anionicity is a further advantage of the fibers of the present invention over those prepared according to the past art, as the polycarboxylic acid crosslinked fibers should be more receptive to cationic additives important to papermaking. For example, the strength of sheets made from the crosslinked fibers should be recoverable without compromising the bulk enhancement by incorporation of a cationic strength resin.
- The polycarboxylic acid crosslinking reaction did not appear to damage the NSWK fibers. Thus, the average fiber length was not changed by the crosslinking reaction. Furthermore, the integrity of the fibers was unchanged by the crosslinking reaction as evidenced by microscopic examination (compare Figures 4 and 5). There was some brightness reduction due to the crosslinking reaction (see Table 1).
- The successful achievement of bulking fibers is by no means limited to crosslinking with citric acid. Any polycarboxylic acid known to crosslink cellulose will work. To demonstrate this, NSWK fibers were crosslinked with butanetetracarboxylic acid according to the method described in Example 1. The resultant fibers, hereafter referred to as "BTATC", were then made into handsheets according to the method described in Example 3. The physical data on these sheets are provided in Table 3. The existence of ester bonds between cellulose and butanetetracarboxylic acid was verified by IR spectroscopy. As can be calculated from the data in Table 3, a 25% incorporation of the BTATC fibers in the NSWK furnish results in a 92% increase in pressed sheet bulk. Furthermore, there was no brightness loss seen in the preparation of the BTATC fibers.
-
- Partial neutralization of the citric acid prior to fiber treatment is not necessary (See Example 4) for the successful preparation of high bulking resilient fibers as described above. Thus, Examples 1-3 were repeated without the use of sodium hydroxide in the preparation of the treatment solution, and the resultant fibers (i.e. CAT fibers) had equivalent performance to that of the CATC fibers (compare data in Table 4 with that in Table 1). Since the citric acid treated fibers were at 25% consistency prior to drying, 39% of available citric acid (i.e. that acid contained in the dry fiber prior to curing) had reacted with the NSWK fibers to produce the CAT fibers described in this example.
- Example 10 was repeated except a treatment solution containing only 5 wt% citric acid was used. As can be seen in Table 4, comparable bulking performance is observed with the resultant CAT fibers relative to those prepared with solutions having twice the level of citric acid. Furthermore, there is a marked improvement in brightness accompanying the reduction of citric acid in the treatment bath. It should also be noted that 53% of the available citric acid had reacted with the NSWK fibers to produce the CAT fibers described in this example.
- Example 10 was repeated except a 3 wt% aqueous solution of citric acid was used for the treatment. As can be seen in Table 4, there was a slight reduction in the bulking ability realized with the CAT fiber prepared under these conditions. Nevertheless, a 2% bulk enhancement is predicted for every 1% incorporation of these fibers in a NSWK furnish. Furthermore, essentially no reduction in brightness was observed with these fibers relative to the control. As was the case with the CAT fibers prepared according to Example 10, about 39% of the available citric acid had reacted with the NSWK fibers to produce the CAT fibers described in this example.
- The percent bound citric acid levels as determined by titration (7) are consistently lower than those determined by ion chromatography. The latter method is considered to be more reliable as it is not predicated on an assumption of the number of active equivalents of carboxyl functionality during base hydrolysis.
- The results of the above examples suggest that the bulking resilient fibers can be obtained using dilute solutions of polycarboxylic acids without the involvement of other chemical additives. Such a simple treatment chemistry greatly enhances the attractiveness of the present invention. Nevertheless, it has been demonstrated by others that certain additives, such as sodium dihydrogen phosphate or sodium hypophosphite, can apparently accelerate the reaction of polycarboxylic acids with cotton fibers. Text. Chem. Color. (1989), 21, 2,13. Such acceleration is useful for the present invention, as shown in Example 13.
-
- NSWK fibers were dispersed in an aqueous solution that contains 5% citric acid and 5% monosodium phosphate, filtered to about 25% consistency, fluff dried, and cured at 180° for 90 seconds. As can be seen in Table 5, the resultant fibers (PCAT) are extremely bulking. The amount of bound citric acid reached in this catalyzed system was 69% of that available. The effectiveness of the monosodium phosphate to accelerate reaction of citric acid with fiber is further exemplified by the observation of 1% bound citric acid after fluff drying alone (PCATU). No bound citric acid has been observed during fluff drying of fibers treated with only citric acid. Some covalently bound phosphate was also detected by ion chromatographic analysis of hydrosylate of PCAT fibers. Thus, phosphate appears to be coreacting along with citric acid, with the cellulose.
- The citric acid crosslinking treatment is effective at producing bulk and resiliency enhancement in a wide variety of wood pulps. Different wood pulps were treated according to Example 13, unless otherwise stated, and made into pressed 65 g/m² handsheets. The bulk data is provided in Table 6.
Table 6 Citric acid crosslinking of different wood pulps Furnisha Bulk (cm³/g) 100% Husumb (predried) 1.8 100% Husumb (never dried) 1.6 100% SSWKc (never dried) 1.8 100% Secondary fibersd 1.8 25% Treated Husum (predried)e 2.3k 25% Treated Husum (never dried)f 2.6k 25% Treated SSWKg , l 2.4 25% Treated secondary fibersh 2.3k 25% Treated CTMPi , m 2.8k 25% CTMP (never dried) 2.2 100% NSWK (never dried)j 1.6 a) Made into pressed 65 g/m² British handsheets b) Scandinavian bleached spruce kraft pulp (untreated) c) Southern pine bleached kraft pulp (untreated) d) Long fiber fraction of Ponderosa secondary fibers (untreated) e) 75% untreated predried Husum f) 75% untreated never dried Husum g) 75% untreated SSWK h) 75% untreated secondary fibers i) 75% NSWK j) Never dried Northern softwood bleached kraft pulp (untreated) k) Some nits present l) Dried and cured with superheated steam at 180°C for 30 seconds m) Starting CTMP was never dried
Claims (33)
- Individualized crosslinked wood pulp cellulosic fibers having intra-fiber chemical bonds characterised in that the intra-fiber crosslink bonds derive from a polycarboxylic acid, and the degree of crosslinking is at least that sufficient to induce in said individualized fibers at least one of the following, namely twisting, curling and resilient bulking tendency.
- Individualized wood pulp cellulosic fibers as claimed in claim 1 crosslinked by from 1 mole % to 25 mole %, calculated on a cellulosic anhydroglucose molar basis, of a polycarboxylic acid crosslinking agent.
- Individualized crosslinked wood pulp cellulosic fibers as claimed in claim 1 or claim 2 in which the polycarboxylic acid crosslinking agent is selected from citric acid and butanetetracarboxylic acid.
- A resilient fibrous bulking pulp comprising individualized crosslinked wood pulp cellulosic fibers as claimed in any one of claims 1 to 3.
- A method for preparing resilient bulking fibers by:(a) contacting wood pulp cellulosic fibers with a crosslinking agent;(b) individualizing the cellulosic fibers; and(c) curing the individualized cellulosic fibers to form intra-fiber cross-link bonds derived from said crosslinking agent between cellulose molecules within individual fibers of the cellulosic fibers,characterised in that a polycarboxylic acid is employed as the crosslinking agent.
- A method as claimed in claim 5 wherein step (a) comprises mixing wood pulp cellulosic fibers with an aqueous solution of polycarboxylic acid.
- A method as claimed in claim 6 wherein step (b) comprises dewatering and drying the fibers.
- A method as claimed in claim 7 wherein the dewatering comprises pressing the cellulosic fibers through a screw press.
- A method as claimed in claim 7 or claim 8 wherein the fibers are dewatered to a consistency of at least 30%.
- A method as claimed in any one of claims 7 to 9 wherein the drying comprises fluff drying.
- A method as claimed in claims 10 wherein the fluff drying is effected with hot gases.
- A method as claimed in any one of claims 7 to 11 wherein the drying is effected with superheated steam.
- A method as claimed in any one of claims 7 to 12 wherein the drying is effected in a reducing atmosphere.
- A method as claimed in any one of claims 7 to 9 wherein the drying step comprises azeotropically drying the fibers in a solvent.
- A method as claimed in any one of claims 7 to 14 wherein the drying step is performed at a temperature lower than that used for curing in step (c).
- The method of any one of claims 5 to 15 wherein the curing is effected in a reducing atmosphere.
- The method of any one of claims 6 to 16 wherein said aqueous solution is 3-10% aqueous solution of a polycarboxylic acid.
- The method of any one of claims 6 to 17 wherein the aqueous solution includes a caustic agent.
- The method of any one of claims 6 to 17 wherein said aqueous solution comprises citric acid and a coreactant/accelerator.
- The method of claim 19 wherein said coreactant/accelerator is selected from phosphates, phosphites, hypophosphites, pyrophosphates and metaphosphates.
- The method of claim 20 wherein the coreactant/accelerator is monosodium phosphate.
- The method of any one of claims 5 to 21 wherein said polycarboxylic acid is selected from citric acid and butanetetracarboxylic acid.
- The method of any one of claims 5 to 22 wherein the curing step comprises heating the fibers at a temperature in the range of from 150 to 180°C for a time period in the range of from 0.5 to 10 minutes.
- The method of any one of claims 5 to 23 wherein said wood pulp fibers are selected from chemical thermal mechanical pulps, Southern and Northern softwood bleached Waft pulps, and secondary fibers.
- The method of any one of claims 5 to 24 wherein said wood pulp fibers are never-dried fibers.
- An improved resilient bulking and absorbent paper product comprising crosslinked wood pulp cellulose fibers to provide improved bulking and absorbent properties, characterised in that the crosslinked fibers are as claimed in any one of claims 1 to 3 or are obtained by a method as claimed in any one of claims 5 to 25.
- A paper product as claimed in claim 26 wherein said crosslinked wood pulp cellulose fibers contain both intra-fiber and interfiber bonds.
- The paper product of claim 26 or 27 further comprising non-crosslinked fibers.
- The paper product of claim 28 wherein said non-crosslinked fibers comprise the majority of said product, on a weight basis.
- The paper product of claim 28 or claim 29 wherein said non-crosslinked fibers are wood fibers.
- The paper product of claim 30 wherein said wood fibers are selected from predried or never dried Scandinavian bleached spruce kraft, Southern pine bleached kraft, secondary fibers, Southern softwood kraft, Northern softwood kraft, and never dried Northern softwood bleached kraft.
- The paper product of any one of claims 26 to 31 wherein said paper product is selected from handsheets, towels, tissues, filters, paperboard, diapers, sanitary napkins, and hospital dressings.
- The use of polycarboxylic acid as the cross-linking agent to induce twisting and curling in individualized wood pulp cellulosic fibers by the formation of intra-fiber crosslink bonds.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US47340490A | 1990-02-01 | 1990-02-01 | |
| US473404 | 1990-02-01 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0440472A1 EP0440472A1 (en) | 1991-08-07 |
| EP0440472B1 true EP0440472B1 (en) | 1995-08-16 |
Family
ID=23879389
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP91300760A Expired - Lifetime EP0440472B1 (en) | 1990-02-01 | 1991-01-31 | High bulking resilient fibers through cross linking of wood pulp fibers with polycarboxylic acids |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP0440472B1 (en) |
| AT (1) | ATE126556T1 (en) |
| CA (1) | CA2035402A1 (en) |
| DE (1) | DE69112089T2 (en) |
| ES (1) | ES2075339T3 (en) |
| FI (1) | FI910467A (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0484101A2 (en) * | 1990-10-31 | 1992-05-06 | James River Corporation Of Virginia | Paper towels having bulky layer |
| US5137537A (en) * | 1989-11-07 | 1992-08-11 | The Procter & Gamble Cellulose Company | Absorbent structure containing individualized, polycarboxylic acid crosslinked wood pulp cellulose fibers |
| US5147345A (en) * | 1991-08-12 | 1992-09-15 | The Procter & Gamble Company | High efficiency absorbent articles for incontinence management |
| US5183707A (en) * | 1989-11-07 | 1993-02-02 | The Procter & Gamble Cellulose Company | Individualized, polycarboxylic acid crosslinked fibers |
| US5190563A (en) * | 1989-11-07 | 1993-03-02 | The Proctor & Gamble Co. | Process for preparing individualized, polycarboxylic acid crosslinked fibers |
| US5199953A (en) * | 1990-09-14 | 1993-04-06 | Ortec, Inc. | Process for reducing discoloration of cellulosic fibers, treated at a high temperature with a solution of a polycarboxylic acid and boric acid or borate |
| US5217445A (en) * | 1990-01-23 | 1993-06-08 | The Procter & Gamble Company | Absorbent structures containing superabsorbent material and web of wetlaid stiffened fibers |
| WO1993014264A1 (en) * | 1992-01-13 | 1993-07-22 | Weyerhaeuser Company | Method and apparatus for crosslinking individualized cellulose fibers |
| US5234423A (en) * | 1991-06-13 | 1993-08-10 | The Procter & Gamble Company | Absorbent article with elastic waist feature and enhanced absorbency |
| US5300192A (en) * | 1992-08-17 | 1994-04-05 | Weyerhaeuser Company | Wet laid fiber sheet manufacturing with reactivatable binders for binding particles to fibers |
| US5308896A (en) * | 1992-08-17 | 1994-05-03 | Weyerhaeuser Company | Particle binders for high bulk fibers |
| US5324391A (en) * | 1990-10-31 | 1994-06-28 | Weyerhaeuser Company | Method for crosslinking cellulose fibers |
| US5352480A (en) * | 1992-08-17 | 1994-10-04 | Weyerhaeuser Company | Method for binding particles to fibers using reactivatable binders |
| US5387207A (en) * | 1991-08-12 | 1995-02-07 | The Procter & Gamble Company | Thin-unit-wet absorbent foam materials for aqueous body fluids and process for making same |
| US5531728A (en) * | 1990-01-23 | 1996-07-02 | The Procter & Gamble Company | Absorbent structures containing thermally-bonded stiffened fibers and superabsorbent material |
| US5556976A (en) * | 1987-01-20 | 1996-09-17 | Jewell; Richard A. | Reactive cyclic N-sulfatoimides and cellulose crosslinked with the imides |
| US5840787A (en) * | 1994-03-25 | 1998-11-24 | Weyerhaeuser Company | Cellulosic products using high-bulk cellulosic fibers |
| US5906894A (en) * | 1994-03-25 | 1999-05-25 | Weyerhaeuser Company | Multi-ply cellulosic products using high-bulk cellulosic fibers |
| US5998511A (en) * | 1994-03-25 | 1999-12-07 | Weyerhaeuser Company | Polymeric polycarboxylic acid crosslinked cellulosic fibers |
| US6020536A (en) * | 1996-06-28 | 2000-02-01 | Sca Hygiene Products Ab | Absorbent body for absorbent articles |
| US6184271B1 (en) | 1994-03-25 | 2001-02-06 | Weyerhaeuser Company | Absorbent composite containing polymaleic acid crosslinked cellulosic fibers |
| US6306251B1 (en) | 1994-03-25 | 2001-10-23 | Weyerhaeuser Company | Multi-ply cellulosic products using high-bulk cellulosic fibers |
| US6340411B1 (en) | 1992-08-17 | 2002-01-22 | Weyerhaeuser Company | Fibrous product containing densifying agent |
| US6379499B1 (en) | 1999-09-28 | 2002-04-30 | University Of Georgia Research Foundation, Inc. | Polymer-aldehyde additives to improve paper properties |
| US6391453B1 (en) | 1992-08-17 | 2002-05-21 | Weyernaeuser Company | Binder treated particles |
| US6395395B1 (en) | 1992-08-17 | 2002-05-28 | Weyerhaeuser Company | Method and compositions for enhancing blood absorbence by superabsorbent materials |
| US6461553B1 (en) | 1992-08-17 | 2002-10-08 | Weyerhaeuser | Method of binding binder treated particles to fibers |
| US6524653B1 (en) | 2000-11-01 | 2003-02-25 | Niponi, Llc | Cellulose-based fire retardant composition |
| US6533989B1 (en) | 2000-08-03 | 2003-03-18 | Kimberly-Clark Worldwide, Inc. | Multi-chamber process and apparatus for forming a stabilized absorbent web |
| US6533978B1 (en) | 2000-08-03 | 2003-03-18 | Kimberly-Clark Worldwide, Inc. | Process and apparatus for forming a stabilized absorbent web |
| US6627041B2 (en) | 2000-03-06 | 2003-09-30 | Georgia-Pacific Corporation | Method of bleaching and providing papermaking fibers with durable curl |
| US6695950B1 (en) | 1999-08-17 | 2004-02-24 | National Starch And Chemical Investment Holding Corporation | Aldehyde modified cellulose pulp for the preparation of high strength paper products |
| WO2015114520A1 (en) * | 2014-01-31 | 2015-08-06 | Kimberly-Clark Worldwide, Inc. | Tissue having reduced hydrogen bonding |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5384011A (en) * | 1993-02-12 | 1995-01-24 | James River Corporation Of Virginia | Process for crosslinking of cellulosic fibers |
| US5384012A (en) * | 1993-02-12 | 1995-01-24 | James River Corporation Of Virginia | Process for crosslinking of cellulosic fibers |
| US5562740A (en) * | 1995-06-15 | 1996-10-08 | The Procter & Gamble Company | Process for preparing reduced odor and improved brightness individualized, polycarboxylic acid crosslinked fibers |
| US6899790B2 (en) | 2000-03-06 | 2005-05-31 | Georgia-Pacific Corporation | Method of providing papermaking fibers with durable curl |
| US6809231B2 (en) * | 2001-11-21 | 2004-10-26 | The United States Of America As Represented By The Secretary Of Agriculture | Flexible and absorbent alginate wound dressing |
| WO2003062818A1 (en) * | 2002-01-23 | 2003-07-31 | Lenzing Aktiengesellschaft | Method for the spectroscopic analysis of viscose constituents |
| US20040058605A1 (en) * | 2002-09-19 | 2004-03-25 | Hansen Michael R. | Polysaccharide treated cellulose fibers |
| US7147446B2 (en) | 2003-01-02 | 2006-12-12 | Weyerhaeuser Company | Crosslinking agent application method and system |
| US7513973B2 (en) | 2004-03-31 | 2009-04-07 | Weyerhaeuser Nr Company | Bleached polyacrylic acid crosslinked cellulosic fibers |
| EP2108676B1 (en) | 2008-04-03 | 2017-12-27 | OrganoClick AB | Crosslinked paper based material |
| EP2309059A1 (en) * | 2009-10-02 | 2011-04-13 | Organoclick Aktiebolag | Method of improving properties of cellulose-based fibrous sheet-formed materials |
| US8980054B2 (en) * | 2012-12-26 | 2015-03-17 | Kimberly-Clark Worldwide, Inc. | Soft tissue having reduced hydrogen bonding |
| US9416494B2 (en) | 2012-12-26 | 2016-08-16 | Kimberly-Clark Worldwide, Inc. | Modified cellulosic fibers having reduced hydrogen bonding |
| US9410292B2 (en) | 2012-12-26 | 2016-08-09 | Kimberly-Clark Worldwide, Inc. | Multilayered tissue having reduced hydrogen bonding |
| EP3277240B1 (en) * | 2015-04-03 | 2020-01-08 | Resolute FP US Inc. | Methods for producing a cellulosic fiber having a high curl index and acquisition |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2759787A (en) | 1953-05-15 | 1956-08-21 | Eastman Kodak Co | Cellulose citrates and their preparation |
| US2987434A (en) * | 1958-09-09 | 1961-06-06 | Paper Chemistry Inst | Method of making pulp |
| BE593301A (en) | 1959-07-24 | |||
| US3224926A (en) | 1962-06-22 | 1965-12-21 | Kimberly Clark Co | Method of forming cross-linked cellulosic fibers and product thereof |
| US3440135A (en) | 1965-12-13 | 1969-04-22 | Kimberly Clark Co | Process for crosslinking cellulosic fibers during gas suspension of fibers |
| US3526048A (en) | 1967-06-07 | 1970-09-01 | Us Agriculture | Cellulose fibers cross-linked and esterified with polycarboxylic acids |
| US3756913A (en) | 1971-06-18 | 1973-09-04 | Scott Paper Co | Modified cellulosic fibers and products containing said fibers |
| US3819470A (en) | 1971-06-18 | 1974-06-25 | Scott Paper Co | Modified cellulosic fibers and method for preparation thereof |
| US4431481A (en) * | 1982-03-29 | 1984-02-14 | Scott Paper Co. | Modified cellulosic fibers and method for preparation thereof |
| US4748076A (en) * | 1985-02-16 | 1988-05-31 | Hayashikane Shipbuilding & Engineering Co., Ltd. | Water absorbent fibrous product and a method of producing the same |
| JPS6233874A (en) | 1985-08-02 | 1987-02-13 | スコツト・ペ−パ−・カンパニ− | Production of modified cellulose fiber |
| US4822453A (en) | 1986-06-27 | 1989-04-18 | The Procter & Gamble Cellulose Company | Absorbent structure containing individualized, crosslinked fibers |
| US4853086A (en) | 1986-12-15 | 1989-08-01 | Weyerhaeuser Company | Hydrophilic cellulose product and method of its manufacture |
| US4820307A (en) | 1988-06-16 | 1989-04-11 | The United States Of America As Represented By The Secretary Of Agriculture | Catalysts and processes for formaldehyde-free durable press finishing of cotton textiles with polycarboxylic acids |
-
1991
- 1991-01-31 ES ES91300760T patent/ES2075339T3/en not_active Expired - Lifetime
- 1991-01-31 AT AT91300760T patent/ATE126556T1/en not_active IP Right Cessation
- 1991-01-31 DE DE69112089T patent/DE69112089T2/en not_active Expired - Fee Related
- 1991-01-31 FI FI910467A patent/FI910467A/en not_active Application Discontinuation
- 1991-01-31 CA CA002035402A patent/CA2035402A1/en not_active Abandoned
- 1991-01-31 EP EP91300760A patent/EP0440472B1/en not_active Expired - Lifetime
Cited By (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5556976A (en) * | 1987-01-20 | 1996-09-17 | Jewell; Richard A. | Reactive cyclic N-sulfatoimides and cellulose crosslinked with the imides |
| US5437418A (en) * | 1987-01-20 | 1995-08-01 | Weyerhaeuser Company | Apparatus for crosslinking individualized cellulose fibers |
| US6436231B1 (en) | 1987-01-20 | 2002-08-20 | Weyerhaeuser | Method and apparatus for crosslinking individualized cellulose fibers |
| US5137537A (en) * | 1989-11-07 | 1992-08-11 | The Procter & Gamble Cellulose Company | Absorbent structure containing individualized, polycarboxylic acid crosslinked wood pulp cellulose fibers |
| US5183707A (en) * | 1989-11-07 | 1993-02-02 | The Procter & Gamble Cellulose Company | Individualized, polycarboxylic acid crosslinked fibers |
| US5190563A (en) * | 1989-11-07 | 1993-03-02 | The Proctor & Gamble Co. | Process for preparing individualized, polycarboxylic acid crosslinked fibers |
| US5531728A (en) * | 1990-01-23 | 1996-07-02 | The Procter & Gamble Company | Absorbent structures containing thermally-bonded stiffened fibers and superabsorbent material |
| US5217445A (en) * | 1990-01-23 | 1993-06-08 | The Procter & Gamble Company | Absorbent structures containing superabsorbent material and web of wetlaid stiffened fibers |
| US5199953A (en) * | 1990-09-14 | 1993-04-06 | Ortec, Inc. | Process for reducing discoloration of cellulosic fibers, treated at a high temperature with a solution of a polycarboxylic acid and boric acid or borate |
| US5324391A (en) * | 1990-10-31 | 1994-06-28 | Weyerhaeuser Company | Method for crosslinking cellulose fibers |
| EP0484101A2 (en) * | 1990-10-31 | 1992-05-06 | James River Corporation Of Virginia | Paper towels having bulky layer |
| EP0484101A3 (en) * | 1990-10-31 | 1992-07-22 | James River Corporation Of Virginia | Paper towels having bulky inner layer |
| US5234423A (en) * | 1991-06-13 | 1993-08-10 | The Procter & Gamble Company | Absorbent article with elastic waist feature and enhanced absorbency |
| US5318554A (en) * | 1991-08-12 | 1994-06-07 | The Procter & Gamble Company | High efficiency absorbent articles for incontinence management |
| US5387207A (en) * | 1991-08-12 | 1995-02-07 | The Procter & Gamble Company | Thin-unit-wet absorbent foam materials for aqueous body fluids and process for making same |
| US5147345A (en) * | 1991-08-12 | 1992-09-15 | The Procter & Gamble Company | High efficiency absorbent articles for incontinence management |
| WO1993014264A1 (en) * | 1992-01-13 | 1993-07-22 | Weyerhaeuser Company | Method and apparatus for crosslinking individualized cellulose fibers |
| US5352480A (en) * | 1992-08-17 | 1994-10-04 | Weyerhaeuser Company | Method for binding particles to fibers using reactivatable binders |
| US5447977A (en) * | 1992-08-17 | 1995-09-05 | Weyerhaeuser Company | Particle binders for high bulk fibers |
| US6425979B1 (en) | 1992-08-17 | 2002-07-30 | Weyerhaeuser Company | Method for making superabsorbent containing diapers |
| US6627249B2 (en) | 1992-08-17 | 2003-09-30 | Weyerhaeuser Company | Method of enhancing blood absorbence by superabsorbent material |
| US6596103B1 (en) | 1992-08-17 | 2003-07-22 | Weyerhaeuser Company | Method of binding binder treated particles to fibers |
| US6521087B2 (en) | 1992-08-17 | 2003-02-18 | Weyerhaeuser Company | Method for forming a diaper |
| US6521339B1 (en) | 1992-08-17 | 2003-02-18 | Weyerhaeuser Company | Diol treated particles combined with fibers |
| US5308896A (en) * | 1992-08-17 | 1994-05-03 | Weyerhaeuser Company | Particle binders for high bulk fibers |
| US6461553B1 (en) | 1992-08-17 | 2002-10-08 | Weyerhaeuser | Method of binding binder treated particles to fibers |
| US6340411B1 (en) | 1992-08-17 | 2002-01-22 | Weyerhaeuser Company | Fibrous product containing densifying agent |
| US5300192A (en) * | 1992-08-17 | 1994-04-05 | Weyerhaeuser Company | Wet laid fiber sheet manufacturing with reactivatable binders for binding particles to fibers |
| US6391453B1 (en) | 1992-08-17 | 2002-05-21 | Weyernaeuser Company | Binder treated particles |
| US6395395B1 (en) | 1992-08-17 | 2002-05-28 | Weyerhaeuser Company | Method and compositions for enhancing blood absorbence by superabsorbent materials |
| US6184271B1 (en) | 1994-03-25 | 2001-02-06 | Weyerhaeuser Company | Absorbent composite containing polymaleic acid crosslinked cellulosic fibers |
| US6620865B2 (en) | 1994-03-25 | 2003-09-16 | Weyerhaeuser Company | Polycarboxylic acid crosslinked cellulosic fibers |
| US6306251B1 (en) | 1994-03-25 | 2001-10-23 | Weyerhaeuser Company | Multi-ply cellulosic products using high-bulk cellulosic fibers |
| US6716306B2 (en) | 1994-03-25 | 2004-04-06 | Weyerhaeuser Company | High bulk cellulose fibers crosslinked with tartaric acid and method of making same |
| US5998511A (en) * | 1994-03-25 | 1999-12-07 | Weyerhaeuser Company | Polymeric polycarboxylic acid crosslinked cellulosic fibers |
| US5840787A (en) * | 1994-03-25 | 1998-11-24 | Weyerhaeuser Company | Cellulosic products using high-bulk cellulosic fibers |
| US5906894A (en) * | 1994-03-25 | 1999-05-25 | Weyerhaeuser Company | Multi-ply cellulosic products using high-bulk cellulosic fibers |
| US6582553B2 (en) | 1994-03-25 | 2003-06-24 | Weyerhaeuser Company | High bulk cellulosic fibers crosslinked with malic acid and process for making the same |
| US6020536A (en) * | 1996-06-28 | 2000-02-01 | Sca Hygiene Products Ab | Absorbent body for absorbent articles |
| US6695950B1 (en) | 1999-08-17 | 2004-02-24 | National Starch And Chemical Investment Holding Corporation | Aldehyde modified cellulose pulp for the preparation of high strength paper products |
| US6379499B1 (en) | 1999-09-28 | 2002-04-30 | University Of Georgia Research Foundation, Inc. | Polymer-aldehyde additives to improve paper properties |
| US6627041B2 (en) | 2000-03-06 | 2003-09-30 | Georgia-Pacific Corporation | Method of bleaching and providing papermaking fibers with durable curl |
| US6533978B1 (en) | 2000-08-03 | 2003-03-18 | Kimberly-Clark Worldwide, Inc. | Process and apparatus for forming a stabilized absorbent web |
| US6533989B1 (en) | 2000-08-03 | 2003-03-18 | Kimberly-Clark Worldwide, Inc. | Multi-chamber process and apparatus for forming a stabilized absorbent web |
| US6524653B1 (en) | 2000-11-01 | 2003-02-25 | Niponi, Llc | Cellulose-based fire retardant composition |
| US6673266B2 (en) | 2000-11-01 | 2004-01-06 | Niponi, Llc | Fire-retardant petroleum composition |
| WO2015114520A1 (en) * | 2014-01-31 | 2015-08-06 | Kimberly-Clark Worldwide, Inc. | Tissue having reduced hydrogen bonding |
| US9127408B2 (en) | 2014-01-31 | 2015-09-08 | Kimberly-Clark Worldwide, Inc. | Tissue having reduced hydrogen bonding |
| GB2537575A (en) * | 2014-01-31 | 2016-10-19 | Kimberly Clark Co | Tissue having reduced hydrogen bonding |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE126556T1 (en) | 1995-09-15 |
| EP0440472A1 (en) | 1991-08-07 |
| ES2075339T3 (en) | 1995-10-01 |
| FI910467A (en) | 1991-08-02 |
| DE69112089T2 (en) | 1996-01-11 |
| CA2035402A1 (en) | 1991-08-02 |
| FI910467A0 (en) | 1991-01-31 |
| DE69112089D1 (en) | 1995-09-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0440472B1 (en) | High bulking resilient fibers through cross linking of wood pulp fibers with polycarboxylic acids | |
| US6592717B2 (en) | Carboxylated cellulosic fibrous web and method of making the same | |
| US6171441B1 (en) | Resin-treated mercerized fibers and products thereof | |
| US6300259B1 (en) | Crosslinkable cellulosic fibrous product | |
| US4431481A (en) | Modified cellulosic fibers and method for preparation thereof | |
| US5755828A (en) | Method and composition for increasing the strength of compositions containing high-bulk fibers | |
| US7018511B2 (en) | Crossed-linked pulp and method of making same | |
| AU2009200305B2 (en) | Treated cellulosic fibers and absorbent articles made from them | |
| EP0752028B1 (en) | Cellulosic products using high-bulk cellulosic fibers | |
| KR100504221B1 (en) | Individualized, chemically crosslinked cellulosic fibers | |
| WO1998013545A1 (en) | Polyanhydride cross-linked fibrous cellulosic products and process for their preparation | |
| CA2155524C (en) | Process for crosslinking of cellulosic fibers | |
| EP2206523B1 (en) | Treated cellulosic fibers and absorbent articles made from them | |
| US6488809B1 (en) | Resin-treated mercerized fibers and products thereof | |
| CA1090060A (en) | Vapor modified cellulosic fibers |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB GR IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19920123 |
|
| K1C3 | Correction of patent application (complete document) published |
Effective date: 19910807 |
|
| 17Q | First examination report despatched |
Effective date: 19930622 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE DK ES FR GB GR IT LI LU NL SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19950816 Ref country code: LI Effective date: 19950816 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19950816 Ref country code: DK Effective date: 19950816 Ref country code: CH Effective date: 19950816 Ref country code: BE Effective date: 19950816 Ref country code: AT Effective date: 19950816 |
|
| REF | Corresponds to: |
Ref document number: 126556 Country of ref document: AT Date of ref document: 19950915 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed |
Owner name: ING. A. GIAMBROCONO & C. S.R.L. |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: JAMES RIVER CORPORATION OF VIRGINIA |
|
| REF | Corresponds to: |
Ref document number: 69112089 Country of ref document: DE Date of ref document: 19950921 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2075339 Country of ref document: ES Kind code of ref document: T3 |
|
| ET | Fr: translation filed | ||
| NLT2 | Nl: modifications (of names), taken from the european patent patent bulletin |
Owner name: JAMES RIVER CORPORATION OF VIRGINIA |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19951116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19960131 Ref country code: GB Effective date: 19960131 |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF THE APPLICANT RENOUNCES Effective date: 19960201 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19960131 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Effective date: 19960930 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19961001 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 19991102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050131 |