US20030196929A1 - Pharmaceutical kit for migraine headache treatment - Google Patents
Pharmaceutical kit for migraine headache treatment Download PDFInfo
- Publication number
- US20030196929A1 US20030196929A1 US10/125,821 US12582102A US2003196929A1 US 20030196929 A1 US20030196929 A1 US 20030196929A1 US 12582102 A US12582102 A US 12582102A US 2003196929 A1 US2003196929 A1 US 2003196929A1
- Authority
- US
- United States
- Prior art keywords
- kit
- container
- pharmaceutical
- treating migraine
- preparing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D23/00—Details of bottles or jars not otherwise provided for
- B65D23/12—Means for the attachment of smaller articles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D81/00—Containers, packaging elements, or packages, for contents presenting particular transport or storage problems, or adapted to be used for non-packaging purposes after removal of contents
- B65D81/32—Containers, packaging elements, or packages, for contents presenting particular transport or storage problems, or adapted to be used for non-packaging purposes after removal of contents for packaging two or more different materials which must be maintained separate prior to use in admixture
- B65D81/3205—Separate rigid or semi-rigid containers joined to each other at their external surfaces
Abstract
A pharmaceutical kit for treating migraine headaches and method of preparing the kit that includes a therapeutically effective dosage of pharmaceutical compounds used in the treatment of migraine headache. The pharmaceutical kit is manufactured with a predetermined number of dosage units of migraine treating pharmaceutical compounds. Additionally the kit provides all components necessary for the administering of the drugs in a safe and convenient manner. An aspect of the pharmaceutical kit may include injections and nasal sprays that are fast acting for relief of the migraine sufferer. A preferred pharmaceutical kit with only oral dosage units of drugs may be provided as an attachment to a water bottle.
Description
- The present invention broadly relates to a pharmaceutical kit and method of packaging the kit for treating migraine headaches.
- Migraine is an age old disease which has been described and dealt with in various ways throughout history by many cultures and civilization. For example, old English literature described migraine as “Hemicrania”, implying that migraine headache is unilateral in the head. However migraine does not always attack in a unilateral manner. It took several centuries to understand the scientific basis and recognize the wide clinical spectrum of this very common illness.
- As of recent times, in the United States alone, there are 28 million people suffering from migraine. Of that population, approximately 21 million are female and 7 million are male. One in four households has at least one migraine sufferer. Migraine prevalence peaks in the 25 to 55 year age ranges in both genders. In the 1999 HIS (International Headache Society) estimate, 52% of the total population of migraine cases remain undiagnosed. The undiagnosed sufferers are most likely self-medicating with over the counter medications. “Migraine Awareness”, i.e., public knowledge of migraine illness, is much more prevalent in the urban areas than in the interior heartland of the United States, for example.
- Additionally, migraine is the number one cause of lost work days. Migraine sufferers visit emergency rooms and doctor's offices more frequently than non-migraine patients. The total cost of lost work in the United States alone is approximately in the range of 5.6 to 17.2 billion dollars a year. Attendant health care costs rival these figures.
- Despite tremendous economic pressures to develop a “magic bullet” style treatment useful in treating the full range of the illness, no manufacturer has done so. For example, a most popular migraine remedy, Sumatriptan, has been used to treat around 140 million cases. However this drug addresses just one aspect of the complex migraine illness. Subjects who are being treated with Sumatriptan most likely take other drugs, over the counter or prescription, to treat aspects of the disease that Sumatriptan does not address. There remains a need for a more convenient approach to complete migraine treatment wherein all of the components and medications necessary for migraine treatment are provided.
- The pathogenesis of migraine may be summarized by:
- (1) genetic pre-disposition (Almost 90% of migraine sufferers have a family history of the illness);
- (2) Environmental factors such as food, atmospheric and climatic changes, emotional upheavals, physical stress, sleep deprivation, and social aggravations; and
- (3) The “cascade phenomenon of migraine”, i.e., neuro-chemical changes occurring in the brain.
- The most important chemical change in the brain in migraine is the decline in the level of the neurotransmitter serotonin. All modern treatment methods to treat migraine attacks use a generic group of drugs called Triptans. Triptans work by elevating the brain serotonin levels through action at the nerve junctions, i.e., synapses. This triggering event results in the release of a variety of other neuro-peptides and mono-amines around the blood vessels in the meninges, i.e., covering of the brain, and in the brain itself. Some of these important neuro-peptides are bradykinin, prostaglandin. A few of the important mono-amines are dopamine and norepinephrine. Their roles are described in further detail below.
- Migraine is a complex illness in which not only serotonin, but other neuro-peptides are implicated. The acute aseptic, i.e., non-infectious, inflammatory reaction of the meninges which causes the severe headache is mediated through the release of a chemical, prostaglandin. Thus, the common anti-inflammatory drugs like Aspirin, Tylenol, Ibuprofen, Alleve etc. are effective in treating the severe headache in migraine attacks because these drugs antagonize prostaglandins.
- During a migraine attack, there is a release of norepinephrin which accounts for the sense of anxiety, palpitation and high blood pressure in the migraine sufferer. Anxiety relieving drugs like Valium, Xanax and Paxil are often useful in treating migraine.
- Release of another important chemical substance called dopamine explains the nausea and vomiting and the tremendous mood swings in migraine. Reglan (Chlorpropomide) is a dopamine blocking agent and hence is successfully used in the acute stages of migraine to treat accompanying symptoms other than the headache, such as nausea and mood swings.
- The drug manufacturers typically put out one single product in the market and promote it studiously. There are four different Triptan preparations already being marketed and several more are expected soon. However, as mentioned above, a “single drug” approach using Triptan, for example, cannot treat migraine adequately.
- A well experienced migraine doctor skillfully prescribes a combination of drugs to treat acute migraine. Under current state of the art, depending upon the presenting manifestation of a migraine attack, the migraine doctor may prescribe a Triptan, e.g., Imitrex, an anti-prostaglandin drug, e.g., Ibuprofen and an anti-dopamine drug, e.g., Reglan, . Patients, very often, use this “cocktail” approach on their own. Surprisingly, it is not at all uncommon to find doctors prescribing only single drug treatment for migraine, which leaves the patient only with partial relief of headache, leaving them unhappy and seeking other opinions. As described above, to successfully treat a migraine attack, there exists the need to use a combination of several drugs. Yet, no manufacturer has assembled a kit with such a combination.
- This invention comprises a pharmaceutical kit for treating migraine headaches, and a method for preparing the pharmaceutical kit. The preparation is a manufacturing process which includes packaging in a container a plurality of specific compounds used in the treatment of migraine that are serotonin level elevating, prostaglandin inhibiting and dopamine blocking, preferably a therapeutically effective amount of each compound sufficient to treat at least 2 separate migraine headache attacks, and, providing use instructions for the effective administering of the compounds for migraine headache treatment. The invention further comprises useful features that provide advantages to the patient as well as the provider, i.e., doctor and pharmacy. For example, preferably separate packages for each compound are packed in separated compartments within the container.
- Specifically, there is provided a method for preparing a migraine headache treatment which comprises: packaging in a container a therapeutically effective dosage of a serotonin level elevating pharmaceutical compound used in the treatment of migraine; packaging in the container a therapeutically effective dosage of a prostaglandin antagonizing, i.e. inhibiting, pharmaceutical compound used in the treatment of migraine; packaging in the container a therapeutically effective dosage of a dopamine blocking pharmaceutical compound used in the treatment of migraine; including and packaged with said container a vessel of water for use in administering the pharmaceutical compounds which are to be orally administered; and packaging in the container instructions for usage of all of the packaged compounds which are contained in the container.
- The present invention will now be described in more detail by referring to the drawings that accompany the present application. It is noted that in the accompanying drawings like reference numerals are used for describing like and corresponding elements thereof.
- FIG. 1 shows a preferred pharmaceutical kit including injection devices and pharmaceutical compounds of the present invention;
- FIG. 2 shows a preferred container including a nasal spray device and alternative pharmaceutical compounds within the container;
- FIG. 3 shows a preferred pharmaceutical kit including pharmaceutical compounds solely for oral administration;
- FIG. 4 a shows a preferred implementation comprising a water bottle having coupling threads for attaching the pharmaceutical kit container;
- FIG. 4 b shows a side cutaway view of a preferred container having the complete regimen of orally administered, individually packaged pharmaceutical compounds;
- FIG. 4 c shows a top down plane view of the preferred container of FIG. 4b;
- FIG. 5 shows the water bottle coupled to the pharmaceutical kit container; and
- FIG. 6 shows bottom of integrally manufactured water bottle and pharmaceutical kit container with access means.
- An aspect of this invention applies to the preparation of a kit for the treatment of migraine headaches. In a preferred embodiment of this invention predetermined quantities representing a therapeutically effective dosage of several compounds for treatment of a migraine headache are packaged along with additional components such as a vessel of water, alcohol swabs and instructions for use, in a container by a manufacturing process which, preferably, seals the container and its contents into a self-contained, unitary, migraine headache treatment kit. Known pharmaceutical compounds may be provided, however it should be understood that yet to be developed compounds may also be used as they become available.
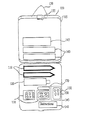
- Preferably, each drug, i.e., pharmaceutical compound, in the kit is packed separately, yet the separately packed drugs are contained in a single, easy to use container, such as the container shown in FIG. 1 100. In a preferred aspect of this pharmaceutical kit for treating migraine headaches,
precise instructions 140 for use of all the constituents are also provided. Additionally, it is preferable that a kit contain enough medications, i.e., pharmaceutical compounds, to treat at least two migraine attacks. As shown in FIG. 1, in a preferred implementation, the container is manufactured such that itscompartments pharmaceutical compounds 132,injection administration devices 110 for drug injection below the skin of a user, and a vessel of water, e.g.,water bottle 120. Sufficient space is also provided for additional components such asalcohol swab packet 170 andinstructions 140. Additionally, the separately packed drugs, i.e., the individually packagedpharmaceutical compounds 132 preferably include labels on the separate packaging identifying the particular pharmaceutical compound contained in the package. Preferably, a container surface such assurface 105 includes alatching device 155 for keeping the contents secure within the container. Additionally, a preferred implementation of this invention includes ahand strap 150 for carrying thekit 100. Although a container with basicallyrectilinear surfaces water bottle 120,injection administration devices 110,alcohol swab packet 170,instructions 140,pharmaceutical package 132, and the like. The container may be made of a relatively soft material such as vinyl and foam, or may provide a relatively hard covering such as high impact plastic. - The container contents include a therapeutically effective dosage of pharmaceutical compounds used in the treatment of migraine, as well as other components to assist drug administration, such as
alcohol swab packet 170.Water bottle 120 is preferably a component that is provided in all implementations of this invention in order to facilitate administering oral dosage units of the pharmaceutical compounds, such as pills, capsules, tablets, and the like. The pharmaceutical compounds are chosen to address various aspects of the migraine condition, such as, e.g., headache, nausea and mood swings. In a preferred implementation of the current invention where all pharmaceutical compounds are oral dosage units, the container may be manufactured to contain more than one water bottle, as shown in FIG. 3 at 120 and 165. - In another preferred implementation of this invention, the provided container is manufactured as a permanent attachment to a water bottle, such as
container 410 attached towater bottle 415 so thatcontainer 410 andwater bottle 415 form one integralmanufactured drug kit 500 as shown in FIG. 5. Thecontainer 410 andwater bottle 415 may be manufactured as an integrated, unitary member, with opening means, such as a sliding door, hinged door, or, as shown in FIG. 6, a revolving cover withhinge pin 610 and a cut outwindow 605, over a bottom ofcontainer 410, for providing access to thecontents 132 of the pharmaceutical kit. - In another preferred implementation of this invention, it is understood that
container 410 is, preferably, cylindrical, having aninner sidewall 409, anouter sidewall 408, and having a depth and size to permit thepharmaceutical compounds 132 andusage instructions 140 to fit inside thecontainer 410, and to permit thecontainer 410 to securely attach, i.e., mate to e.g., thelower portion 417 of preferablycylindrical water bottle 415 by couplinglower portion 417 ofwater bottle 415 andcontainer 410 together such thatlower portion 417 andcontainer 410 are securely held together by friction contact between them. Additionally, the coupling, in a preferred implementation, as shown in FIG. 4b at 413, may be provided by threads, preferably oninner sidewall 409 for threading thecontainer 410 on towater bottle 415.Water bottle 415 comprisescomplementary threads 419 on itslower portion 417 to accommodate threading thecontainer 410 on to thelower portion 417 ofwater bottle 415.Container 410 may also be divided intocompartments 412 for a complete regimen of orally administered, individually packaged pharmaceutical compounds (FIG. 4c 132) such as those discussed below in Migraine Kit-Type III. Preferably, a disk shapedlid 414, withstrap 416 for removal, is included to cover the contents ofcompartments 412, yet permitting the secure attachment ofcontainer 410 withlid 414 towater bottle 415. However, with or without thelid 414, thewater bottle 415 mated tocontainer 410 provides a convenient, self contained migraine headache treatment kit. - Because, as discussed above, the most important chemical change in the brain in migraine is the decline in the level of the neurotransmitter serotonin, it is important to provide in the treatment kit 100 a means for elevating serotonin levels. To that end, preferably, a therapeutically effective dosage of a serotonin level elevating pharmaceutical compound used in the treatment of migraine is provided. Additionally, alternative methods of administering the serotonin elevating compound, such as
injection devices 110 or nasal spray device 210 (shown in FIG. 2), are provided for fastest relief of symptoms associated with lower serotonin levels during a migraine attack. It should be understood that when thenasal spray device 210 is supplied, the container is manufactured such thatcompartment 260 fits neatly around it. When one ormore injection devices 110 are used,alcohol swab packet 170 is provided for skin preparation of the migraine suffered. All modern treatment methods to treat this aspect of a migraine attack use a generic group of drugs called Triptans. One of the most popular Triptan drugs is Imitrex, manufactured by Glaxo Wellcome, now Glaxo Smith Kleine (GSK). Imitrex, i.e., Sumatriptan, is currently marketed in a tablet form (50 mg and 100 mg), a nasal spray form (20 mg) and a 6 mg injectable form, i.e. a statdose pen. In a preferred embodiment of this invention, Sumatriptan may be provided in tablet, as well as liquid forms. - While no skin patch preparation is yet available, it is within the scope of this invention to include a skin patch when a skin patch becomes available. Other available serotonin level elevating compounds which are successfully used and may, preferably, be provided by this invention include Rizatriptan, Zolmitriptan, and Almotriptan. A predetermined number of serotonin level elevating compound dosage units is provided in the manufactured
drug kit 100. - To combat the nausea and moodswings experienced by a large number of migraine sufferers, a dopamine blocking pharmaceutical compound may be prescribed. A preferred implementation of this invention provides a therapeutically effective dosage of dopamine blocking pharmaceutical compound such as Reglan, i.e., Metoclopramide, or Compazine, i.e., Prochlorperazine. A predetermined number of dopamine blocking pharmaceutical compound dosage units is provided in the manufactured
drug kit 100. - Elevated prostaglandin, associated with the severe headache caused by inflamed meninges of migraine sufferers may be combated by lowering, i.e., antagonizing the prostaglandin levels. Thus, preferably a therapeutically effective dosage of a prostaglandin inhibiting pharmaceutical compound, such as, for example, antiinflammatory drugs like Aspirin, i.e., Acetyl Salicylic Acid (325 mg to 500 mg), Tylenol (250 mg to 750 mg), i.e., Acetaminophen, Ibuprofen, and Naproxyn is provided to antagonize, i.e., lower the prostaglandin levels by inhibiting prostaglandin production of the migraine sufferer, wherein relief from the headache is obtained. A predetermined number of prostaglandin inhibiting pharmaceutical compound dosage units is provided in the manufactured
drug kit 100. It should be understood that all of the migraine treatment pharmaceutical compounds discussed above and provided in manufactureddrug kit 100 may also be provided in manufactureddrug kit 500. - Listed below are preferable migraine kit compositions with sufficient dosage units, preferably, to provide treatment of at least two separate migraine attacks. It should be understood that the lists are representative examples and do not limit the scope of the migraine kit compositions.
- Migraine Kit-Type I:
- Imitrex (Sumatriptan) Injection ( Stat Dose Pen) quantity 2;
- Imitrex (Sumatriptan) Tablet 50 mg, quantity 2;
- Reglan (Metoclopromide HCL) 10 mg, quantity 2; and
- Select either Ibuprofen 800 mg, quantity 2 (or)
Naproxyn 500 mg, quantity 2. - Migraine Kit-Type II
- Imitrex (Sumatriptan) Nasal Spray 5 mg to 20 mg, quantity 2
- Imitrex (Sumatriptan) Tablets 50 mg, quantity 2
- Select from either Reglan (Metoclopromide HCL) 5 mg to 10 mg, quantity 2 (or) Prochlorperazine 5 mg to 10 mg, quantity 2
- Select from either Ibuprofen 400 mg to 800 mg, quantity 2 (or)
Naproxyn 500 mg to 750 mg, quantity 2. - Migraine Kit-Type III
- Imitrex (Sumatriptan)
Tablet 100 mg, quantity 2 - Imitrex (Sumatriptan) Tablet 50 mg, quantity 2
- Reglan (Metoclopromide HCL) 10 mg, quantity 2
- Ibuprofen 800 mg, quantity 2 (or)
Naproxyn 500 mg, quantity 2 - In one aspect of this invention, instead of providing the Imitrex tablet in Migraine Kit type III, one of the following compounds maybe provided:
- Maxalt Tablet or MLT, 5 mg to 10 mg (Rizatriptan);
- Zomig Tablet or ZMT, 2.5 mg to 5 mg (Zolmatriptan);
- Axert Tablet 6.25 mg to 12.5 mg (Almotriptan).
- Now that the invention has been described by way of a preferred embodiment, various modifications and improvements will occur to those of skill in the art. For example, a treatment kit of multiple pharmaceutical compounds could be prepared for the treatment of hypertension, congestive heart failure, or asthma. Thus, it should be understood that the preferred embodiment is provided as an example and not as a limitation. The scope of the invention is defined by the appended claims.
Claims (31)
1. A method for preparing a pharmaceutical kit for treating migraine headaches, said method comprising:
packaging in a container a therapeutically effective dosage of a serotonin level elevating pharmaceutical compound used in the treatment of migraine;
packaging in said container a therapeutically effective dosage of a prostaglandin inhibiting pharmaceutical compound used in the treatment of migraine;
packaging in said container a therapeutically effective dosage of a dopamine blocking pharmaceutical compound used in the treatment of migraine;
packaging with said container a vessel of water, said vessel of water having an upper portion and a lower portion, and for use in administering the pharmaceutical compounds which are to be orally administered; and
packaging in said container instructions for usage of the pharmaceutical compounds which it contains wherein a convenient container comprising a plurality of the pharmaceutical compounds for the treatment of migraine headaches is provided.
2. The method for preparing a kit for treating migraine headaches as claimed in claim 1 , wherein said packaging in said container a therapeutically effective dosage of the serotonin level elevating pharmaceutical compound comprises packaging at least one stat dose pen of injectable Sumatriptan in said container.
3. The method for preparing a pharmaceutical kit for treating migraine headaches as claimed in claim 1 , wherein said packaging in said container a therapeutically effective dosage of the serotonin level elevating pharmaceutical compound comprises packaging in said container a predetermined number of dosage units comprising a triptan selected from the group consisting of Sumatriptan, Rizatriptan, Zolmitriptan, and Almotriptan.
4. The method for preparing a kit for treating migraine headaches as claimed in claim 3 , wherein any one of the Sumatriptan dosage units comprises Sumatriptan in the range of 50 mg to 100 mg.
5. The method for preparing a kit for treating migraine headaches as claimed in claim 3 , wherein any one of the Rizatriptan dosage units comprises Rizatriptan in the range of 5 mg to 10 mg.
6. The method for preparing a kit for treating migraine headaches as claimed in claim 3 , wherein any one of the Zolmitriptan dosage units comprises Zolmitriptan in the range of 2.5 mg to 5 mg.
7. The method for preparing a kit for treating migraine headaches as claimed in claim 3 , wherein any one of the Almotriptan dosage units comprises Almotriptan in the range of 6.25 mg to 12.5 mg.
8. The method for preparing a kit for treating migraine headaches as claimed in claim 1 , wherein said packaging in said container a therapeutically effective dosage of the serotonin level elevating pharmaceutical compound comprises packaging a predetermined number of nasal spray devices containing Sumatriptan in said container.
9. The method for preparing a kit for treating migraine headaches as claimed in claim 8 , wherein any one of the Sumatriptan containing nasal spray devices comprises Sumatriptan in the range of 5 mg to 20 mg.
10. The method for preparing a kit for treating migraine headaches as claimed in claim 1 , wherein said packaging in said container a therapeutically effective dosage of the dopamine blocking pharmaceutical compound comprises packaging in said container a predetermined number of dosage units comprising a dopamine blocking pharmaceutical compound selected from the group consisting of Metoclopramide, and Prochlorperazine.
11. The method for preparing a kit for treating migraine headaches as claimed in claim 10 , wherein any one of the Metoclopramide dosage units comprises Metoclopramide in the range of 5 mg to 10 mg.
12. The method for preparing a kit for treating migraine headaches as claimed in claim 10 , wherein any one of the Prochlorperazine dosage units comprises Prochlorperazine in the range of 5 mg to 10 mg.
13. The method for preparing a kit for treating migraine headaches as claimed in claim 1 , wherein said packaging in said container a therapeutically effective dosage of the prostaglandin inhibiting pharmaceutical compound comprises packaging in said container a predetermined number of dosage units comprising a prostaglandin inhibiting pharmaceutical compound selected from the group consisting of Ibuprofen, Naproxyn, Acetaminophen, and Acetylsalicylic Acid.
14. The method for preparing a kit for treating migraine headaches as claimed in claim 13 , wherein any one of the Ibuprofen dosage units comprises Ibuprofen in the range of 400 mg to 800 mg.
15. The method for preparing a kit for treating migraine headaches as claimed in claim 13 , wherein any one of the Naproxyn dosage units comprises Naproxyn in the range of 500 mg to 750 mg.
16. The method for preparing a kit for treating migraine headaches as claimed in claim 13 , wherein any one of the Acetaminophen dosage units comprises Acetaminophen in the range of 250 mg to 750 mg.
17. The method for preparing a kit for treating migraine headaches as claimed in claim 13 , wherein any one of the Acetylsalicylic Acid dosage units comprises Acetylsalicylic Acid in the range of 325 mg to 500 mg.
18. The method for preparing a kit for treating migraine headaches as claimed in claim 1 , wherein said including with said container comprises packaging in said container the vessel of water.
19. The method for preparing a kit for treating migraine headaches as claimed in claim 1 , wherein said including with said container comprises attaching said container to the vessel of water.
20. The method for preparing a kit for treating migraine headaches as claimed in claim 19 , wherein said attaching said container to the vessel of water further comprises coupling said container to the lower portion of the vessel of water.
21. A pharmaceutical kit, manufactured to include a complete regimen of components and pharmaceutical compounds necessary for treating migraine headache comprising:
a) a container including a therapeutically effective dosage of a plurality of pharmaceutical compounds used in the treatment of migraine;
b) a vessel of water packaged with said container, said vessel of water having an upper portion and a lower portion, and being used for administering of the pharmaceutical compounds which are to be orally administered; and
c) instructions for the safe and effective use of the pharmaceutical kit for treating the migraine headache.
22. The pharmaceutical kit for treating migraine headache as claimed in claim 21 , further comprising at least one injection device having a therapeutically effective dosage of Sumatriptan, said injection device being included in said container for injection of said Sumatriptan below the skin of a user.
23. The pharmaceutical kit for treating migraine headache as claimed in claim 22 , further comprising an alcohol swab packet included in said container, said alcohol swab packet being used for preparation of the user's skin associated with the administering of the Sumatriptan which is to be injected.
24. The pharmaceutical kit for treating migraine headache as claimed in claim 21 , further comprising at least one nasal spray device having a therapeutically effective dosage of Sumatriptan, said nasal spray device being included in said container for administering of said Sumatriptan through the nasal passages of a user.
25. The pharmaceutical kit for treating migraine headache as claimed in claim 21 , further comprising separate labeled packaging for each of the plurality of pharmaceutical compounds, said separate labeled packaging identifying the pharmaceutical compound which it contains.
26. The pharmaceutical kit for treating migraine headache as claimed in claim 25 , wherein said plurality of pharmaceutical compounds comprises:
at least two 100 mg oral dosage units of Sumatriptan;
at least two 50 mg oral dosage units of Sumatriptan;
at least two 10 mg oral dosage units of Metoclopromide HCL; and
at least two oral dosage units comprising a prostaglandin inhibiting pharmaceutical compound selected from the group consisting of Ibuprofen and Naproxyn.
27. The pharmaceutical kit for treating migraine headache as claimed in claim 26 , wherein each oral dosage unit of Ibuprofen comprises an 800 mg dose.
28. The pharmaceutical kit for treating migraine headache as claimed in claim 26 , wherein each oral dosage unit of Naproxyn comprises a 500 mg dose.
29. The pharmaceutical kit for treating migraine headache as claimed in claim 2 1, wherein the vessel of water packaged with said container is attached to the container to provide a convenient, self contained migraine headache treatment kit.
30. The pharmaceutical kit for treating migraine headache as claimed in claim 29 , further comprising:
the vessel of water having a cylindrical shape including threading on the lower portion of the vessel of water; and
the container having a cylindrical shape with an outer side wall and an inner side wall, said inner side wall having threading complementary to the threading on the lower portion of the vessel of water, thereby providing a coupling for attaching the container to the vessel of water.
31. The pharmaceutical kit for treating migraine headache as claimed in claim 29 , further wherein the vessel of water attached to the container comprises an integrally manufactured, unitary member having a revolving cover with a cutout window and hinge pin for providing access to the pharmaceutical kit contents.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/125,821 US20030196929A1 (en) | 2002-04-19 | 2002-04-19 | Pharmaceutical kit for migraine headache treatment |
| US11/201,536 US20050284792A1 (en) | 2002-04-19 | 2005-08-11 | Pharmaceutical kit for migraine headache treatment |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/125,821 US20030196929A1 (en) | 2002-04-19 | 2002-04-19 | Pharmaceutical kit for migraine headache treatment |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/201,536 Continuation US20050284792A1 (en) | 2002-04-19 | 2005-08-11 | Pharmaceutical kit for migraine headache treatment |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030196929A1 true US20030196929A1 (en) | 2003-10-23 |
Family
ID=29214857
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/125,821 Abandoned US20030196929A1 (en) | 2002-04-19 | 2002-04-19 | Pharmaceutical kit for migraine headache treatment |
| US11/201,536 Abandoned US20050284792A1 (en) | 2002-04-19 | 2005-08-11 | Pharmaceutical kit for migraine headache treatment |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/201,536 Abandoned US20050284792A1 (en) | 2002-04-19 | 2005-08-11 | Pharmaceutical kit for migraine headache treatment |
Country Status (1)
| Country | Link |
|---|---|
| US (2) | US20030196929A1 (en) |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050224387A1 (en) * | 2004-03-30 | 2005-10-13 | Desjardins Donna L | Kit for promoting a product and related methods |
| FR2896487A1 (en) * | 2006-01-24 | 2007-07-27 | Michel Charles Antoine Magnac | Medicines transporting and storing device for use by e.g. handicapped person, has receptacle in shape of pommel for storing medicines and reservoir containing beverage for taking medicines, where receptacle is screwed on reservoir |
| US20070267321A1 (en) * | 2006-05-17 | 2007-11-22 | Fleming Gayle R | Mobile medicine cabinet and method of use |
| US20080004613A1 (en) * | 2005-05-13 | 2008-01-03 | Benechill, Inc. | Methods and devices for treatment of migraines |
| US20080031959A1 (en) * | 2006-07-28 | 2008-02-07 | Blondino Frank E | Anti-migraine oral spray formulations and methods |
| US20090152159A1 (en) * | 2008-10-28 | 2009-06-18 | William Beeman | Self-contained, portable kit for carrying personal items |
| US20090325999A1 (en) * | 2008-06-27 | 2009-12-31 | Jie Du | Personalized pharmaceutical kits, packaging and compositions for the treatment of allergic conditions |
| US20150027922A1 (en) * | 2012-02-17 | 2015-01-29 | FRESCO Bernard | First-aid kit |
| US20150374918A1 (en) * | 2013-08-19 | 2015-12-31 | Dr. Reddy's Laboratories Ltd. | Selectable single dose auto-injector and methods of making and using same |
| US10561527B2 (en) | 2005-05-13 | 2020-02-18 | Braincool Ab | Methods and devices for non-invasive cerebral and systemic cooling alternating liquid mist/gas for induction and gas for maintenance |
| US10806590B2 (en) * | 2005-06-15 | 2020-10-20 | P Tech, Llc | Methods and systems for providing gender specific pharmaceuticals |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080025018A1 (en) * | 2006-09-22 | 2008-01-31 | Bonni Shevin Sandy | Water bottle-container-flashlight apparatus |
| US8141727B2 (en) * | 2009-01-17 | 2012-03-27 | Patrick Mulligan | Water bottle with dosage in a dispenser cap |
| US8376134B1 (en) | 2012-05-18 | 2013-02-19 | Philip Andrew Underwood | Drink bottle with multiple drink dosage device |
| US20210369704A1 (en) * | 2020-05-29 | 2021-12-02 | Arizona Board Of Regents On Behalf Of The University Of Arizona | Compositions and methods for preventing and treating headache through enhancing 2-arachydonyl glyerol activity |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5891885A (en) * | 1996-10-09 | 1999-04-06 | Algos Pharmaceutical Corporation | Method for treating migraine |
| US6077530A (en) * | 1997-07-28 | 2000-06-20 | Weinstein; Robert | Analgesic dosage units for coordinated administration |
| US20010004644A1 (en) * | 1997-07-21 | 2001-06-21 | Levin Bruce H. | Compositions, kits, apparatus, and methods for inhibiting cephalic inflammation |
-
2002
- 2002-04-19 US US10/125,821 patent/US20030196929A1/en not_active Abandoned
-
2005
- 2005-08-11 US US11/201,536 patent/US20050284792A1/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5891885A (en) * | 1996-10-09 | 1999-04-06 | Algos Pharmaceutical Corporation | Method for treating migraine |
| US20010004644A1 (en) * | 1997-07-21 | 2001-06-21 | Levin Bruce H. | Compositions, kits, apparatus, and methods for inhibiting cephalic inflammation |
| US6077530A (en) * | 1997-07-28 | 2000-06-20 | Weinstein; Robert | Analgesic dosage units for coordinated administration |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050224387A1 (en) * | 2004-03-30 | 2005-10-13 | Desjardins Donna L | Kit for promoting a product and related methods |
| US10561527B2 (en) | 2005-05-13 | 2020-02-18 | Braincool Ab | Methods and devices for non-invasive cerebral and systemic cooling alternating liquid mist/gas for induction and gas for maintenance |
| US20080004613A1 (en) * | 2005-05-13 | 2008-01-03 | Benechill, Inc. | Methods and devices for treatment of migraines |
| US10806590B2 (en) * | 2005-06-15 | 2020-10-20 | P Tech, Llc | Methods and systems for providing gender specific pharmaceuticals |
| FR2896487A1 (en) * | 2006-01-24 | 2007-07-27 | Michel Charles Antoine Magnac | Medicines transporting and storing device for use by e.g. handicapped person, has receptacle in shape of pommel for storing medicines and reservoir containing beverage for taking medicines, where receptacle is screwed on reservoir |
| US20070267321A1 (en) * | 2006-05-17 | 2007-11-22 | Fleming Gayle R | Mobile medicine cabinet and method of use |
| US20080031959A1 (en) * | 2006-07-28 | 2008-02-07 | Blondino Frank E | Anti-migraine oral spray formulations and methods |
| US20090325999A1 (en) * | 2008-06-27 | 2009-12-31 | Jie Du | Personalized pharmaceutical kits, packaging and compositions for the treatment of allergic conditions |
| US7926661B2 (en) * | 2008-10-28 | 2011-04-19 | William Beeman | Self-contained, portable kit for carrying personal items |
| US20090152159A1 (en) * | 2008-10-28 | 2009-06-18 | William Beeman | Self-contained, portable kit for carrying personal items |
| US20150027922A1 (en) * | 2012-02-17 | 2015-01-29 | FRESCO Bernard | First-aid kit |
| US9730845B2 (en) * | 2012-02-17 | 2017-08-15 | Bernard Fresco | First-aid kit |
| US20150374918A1 (en) * | 2013-08-19 | 2015-12-31 | Dr. Reddy's Laboratories Ltd. | Selectable single dose auto-injector and methods of making and using same |
| US9931469B2 (en) | 2013-08-19 | 2018-04-03 | Dr. Reddy's Laboratories Ltd. | Selectable single dose auto-injector and methods of making and using same |
| US9931470B2 (en) * | 2013-08-19 | 2018-04-03 | Dr. Reddy's Laboratories Ltd. | Selectable single dose auto-injector and methods of making and using same |
Also Published As
| Publication number | Publication date |
|---|---|
| US20050284792A1 (en) | 2005-12-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20050284792A1 (en) | Pharmaceutical kit for migraine headache treatment | |
| Erhardt | Cigarette smoking: an undertreated risk factor for cardiovascular disease | |
| US20120021075A1 (en) | Dual-chamber packaging systems for cannabis-infused products systems | |
| Zheng et al. | Metabolic changes associated with second-generation antipsychotic use in Alzheimer’s disease patients: the CATIE-AD study | |
| CN100413494C (en) | Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma | |
| US20080113973A1 (en) | Compositions and methods involving the combination of a thromboxane A2 receptor antagonist and an inhibitor of cyclooxygenase-2 | |
| KR102597910B1 (en) | Method and composition for suppressing symptoms associated with hangover state | |
| US20050053648A1 (en) | Medication delivery device | |
| US20120248004A1 (en) | Method and apparatus for packaging and delivering nutraceutical, pharmaceutical, and polyceutical compositions | |
| Indraratna et al. | Acute ST-elevation myocardial infarction, a unique complication of recreational nitrous oxide use | |
| Renz et al. | Oral antihistamines reduce the side effects from rapid vancomycin infusion | |
| Awang | Prescribing therapeutic feverfew (Tanacetum parthenium (L.) Schultz bip., syn. Chrysanthemum parthenium (L.) Bernh.) | |
| Myrenfors et al. | Moclobemide overdose | |
| Stevenson et al. | Aspirin sensitivity in asthmatics: when may this drug be safe? | |
| Chan et al. | Hong Kong Poison Information Centre: Annual Report 2015 | |
| WO2008014471A1 (en) | Drug combination pharmaceutical compositions and methods for using them | |
| AU2001273932B2 (en) | Drug combination for the treatment of headache comprising mirtazapine and paracetamol or a non-steroidal anti-inflammatory drug | |
| JP2020070285A (en) | Pharmaceutical product | |
| AU2001273932A1 (en) | Drug combination for the treatment of headache comprising mirtazapine and paracetamol or a non-steroidal anti-inflammatory drug | |
| RU2222305C1 (en) | General road first-aid kit | |
| US20060088588A1 (en) | Medication administering device and system | |
| EP3622940B1 (en) | Housing for storing and delivering doses of medicinal products to be administered | |
| Hill et al. | Fenclofenac and soluble aspirin in rheumatoid arthritis: a comparative trial | |
| Mayer | Instability of nitroglycerin tablets | |
| Siddiqui et al. | Shock Due to Minoxidil Toxicity Related to Medication Dispensing Error |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |