US3545671A - Apparatus for and method of collecting,storing,separating and dispensing blood and blood components - Google Patents
Apparatus for and method of collecting,storing,separating and dispensing blood and blood components Download PDFInfo
- Publication number
- US3545671A US3545671A US616005A US3545671DA US3545671A US 3545671 A US3545671 A US 3545671A US 616005 A US616005 A US 616005A US 3545671D A US3545671D A US 3545671DA US 3545671 A US3545671 A US 3545671A
- Authority
- US
- United States
- Prior art keywords
- bag
- blood
- sealed
- plasma
- compartments
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150015—Source of blood
- A61B5/15003—Source of blood for venous or arterial blood
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150206—Construction or design features not otherwise provided for; manufacturing or production; packages; sterilisation of piercing element, piercing device or sampling device
- A61B5/150221—Valves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150206—Construction or design features not otherwise provided for; manufacturing or production; packages; sterilisation of piercing element, piercing device or sampling device
- A61B5/150251—Collection chamber divided into at least two compartments, e.g. for division of samples
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150366—Blood collection bags, e.g. connected to the patient by a catheter comprising means for removing a small sample of collected blood from the bag
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/153—Devices specially adapted for taking samples of venous or arterial blood, e.g. with syringes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/155—Devices specially adapted for continuous or multiple sampling, e.g. at predetermined intervals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/02—Blood transfusion apparatus
- A61M1/029—Separating blood components present in distinct layers in a container, not otherwise provided for
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/15—Devices for taking samples of blood
- A61B5/150007—Details
- A61B5/150206—Construction or design features not otherwise provided for; manufacturing or production; packages; sterilisation of piercing element, piercing device or sampling device
- A61B5/150259—Improved gripping, e.g. with high friction pattern or projections on the housing surface or an ergonometric shape
Definitions
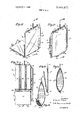
- PATENTED DEC 81970 SHEET l 0F 4 PI IZ Red CcHS Plasma l/Vl/HVIUR EUGENE D- Ross fcfzQamfl d aw;
- This invention relates to the handling and administering of blood and its components and more particularly to an improved apparatus for and method of collecting and storing whole blood, dividing an initially collected quantity of the blood into smaller discrete quantities, separating out such components of the collected whole blood as its red cells, plasma, platelets, leukocytes and the like for separate utilization thereof as may be desired, reconstituting the separated cells and plasma or other components of the blood, and otherwise handling the collected blood, including centrifugation thereof, all of which operations are performed in a single flexible envelop the interior of which is sealed from atmosphere and provides for fractionating the whole blood into separately usable smaller portions or components thereof completely free of any danger of air embolism and under such bacteriologically sterile and aseptic conditions as to practically eliminate any chance of contamination from external sources.
- a further object of the present invention is to provide a single bag closed system for collecting and handling blood which is compact and simple to set up and use, which may be packaged and sterilized at the source of its manufacture as a complete transfusion unit and which is relatively inexpensive to manufacture and economical to use.
- FIG. I is a side elevational view of the closed system single receptacle unit constructed in accordance with and embodying the principles of the present invention, certain portions thereof being shown in section;
- FIG. 2 is a detail sectional view as taken along the line 2-2 of FIG. 1;
- FIG. 3 is a sectional view of the same detail as taken along the line 3-3 of FIG. 2;
- FIG. 4 is a vertical sectional view of the receptacle as taken along the line 4-4 of FIG. 1;
- FIG. 5 is a detail section view as taken along the line 5-5 of FIG. 1;
- F IG. 6 is an elevational view of a clamp adapted to seal portions of the receptacle from one another;
- FIG. 7 is a view showing the clamp of FIG. 6 in its closed clamping condition
- FIG. 8 is an end elevational view of the clamp as viewed from the line 8-8 of FIG. 7;
- FIGS. 9 to 13 inclusive are vertical sectional views each taken along the line 4-4 of FIG. 1 showing the receptacle variously compartmentalized with each compartment filled with a portion of the initially collected blood;
- FIG. 14 is an elevational view showing the receptacle of FIG. 1 horizontally and vertically separated into several blood-receiving compartments;
- FIG. 15 is a perspective view showing the receptacle of FIG. 1 in partially folded condition with the clamp attached to provide two compartments separated along the fold line;
- FIG. 16 is a vertical sectional view of the unit as taken along the line 16-16 ofFIG. 15;
- FIG. 17 is a perspective view of the unit shown in FIG. 15 in its completely folded condition, showing the upper compartment filled with collected blood;
- FIG. 18 is a vertical sectional view of the blood-filled unit as taken along the line 18-18 of FIG. 17;
- FIG. 19 is a vertical sectional view corresponding to FIG. 18 but showing the unfilled portion of the receptacle in rolled-up condition instead of being folded flatwise against the filled portion as in FIG. 18;
- FIG. 20 to 26 inclusive are elevational views illustrating the successive steps employed for fractionating within the receptacle the whole blood initially collected therein as shown in FIGS. 17 and 18 into its component parts of red cells, plasma, platelets and leukocytes;
- FIGS. 27 to 30 inclusive are elevational views illustrating the successive steps of an alternate procedure which may be employed for separating out the red cells, plasma and platelets of the whole blood initially collected and stored in the receptacle.
- the blood-handling ap paratus of the present invention essentially comprises a single receptacle or container in the form of a flexible-walled deformable bladder or bag 10 of generally rectangular outline having its perimetral edges completely sealed and fitted at preselected points with a main port 11 and a plurality of auxiliary ports 12 arranged in spaced relation to the main port and to one another about the marginal edge of the bag.
- the bag 10 may be formed of a single sheet of suitable plastic material which is folded upon itself to provide a pair of juxtaposed panels having their registering free edges heat-sealed together, or it may be formed of a pair of separate plastic sheets having all of their free edges suitably sealed together, as by heat-sealing, the use of adhesives or otherwise.
- the bag 10 is preferably formed of sheet material manufactured of polyvinyl, polypropylene, polyethylene fluorocarbon (e.g. Teflon and Kel-F) derivatives and like thermoplastic resins sufficiently transparent or translucent for visual inspection of the contents of the bag and capable of being heat-sealed together.
- the plastic material additionally should be of medical quality, flexible and sufficiently tough to withstand autoclaving, centrifugation and the extremes of heat and cold to which the filled bag may be subjected during treatment and storage of its contents.
- a further essential characteristic of the bag of the present invention is that it be of a size and capacity substantially greater than that required for receiving and accommodating therein the quantity of whole blood initially collected from the donor.
- the volumetric capacity of the bag should desirable be from 750 to 1,000 cc. in order to effectually carry out the procedures in accordance with principles and objectives of the present invention.
- a flexible tube 13 having its opposite ends 14 and 15 in free communication with the interior of the bag and an intermediate portion connected, as by way of a T-connection 16, to the bag for communication with the bag interior.
- the inner end of the T-connection is normally closed by a removable ball-type seal 17 to prevent passage of material into and out of the bag by way of the T-connection 16 except under certain desired conditions, as will appear more fully hereinafter.
- This ball-type seal or closure member 17 is preferably in the form of a marble of glass, metal or nonporous other material inert to blood having a diameter somewhat greater than the internal diameter of the T-connection tubing so that when it is fitted in the inner end of said tubing the latter is expanded and holds the marble in its sealing position until such time that it is unseated from the tubing by pressure applied against the latter to squeeze the ball out of the tubing and into the cavity of the bag. It will be understood that any other suitable readily openable closure means may be provided in lieu of the ball closure 17 for maintaining the T-connection l6 closed against flow of fluid therethrough until such time as such flow may be desired.
- the tube 13 may be pinched closed adjacent any one of its points of entry into the bag by any suitable clamping means (not shown), which may be removed as required to effect communication by way of the tube between separately sealed portions of the bag.
- the tube 13 with its T-connection 16 may be formed as an integral part of the bag 10, it being only necessary that this tube be substantially as flexible as the material of which the bag is formed.
- the main port 11 and the several auxiliary ports 12 are also formed of flexible tubing having their outer ends each closed by a stopper 19 having a core or diaphragm which may be readily pierced by the needle normally fitted on the tube employed for collecting or dispensing blood and its components.
- auxiliary ports 12 may be employed in certain instances as inlet or entry ports and in other instances as exit or discharge ports and to this end these auxiliary ports are so perimetrally spaced about the bag as to provide at least one port, in each of the several separate zones or compartments into which the bag may be divided as hereinafter described so that each zone or compartment may thereby be provided with an inlet and/or outlet port.
- the bag 10 is provided at each of its opposite ends and upon one of the sidewall panels thereof substantially midway between its opposite ends with a pair of laterally spaced hanger or support elements 20-20 by means of which the bag may be suspended from either end in its fully extended condition or from its middle in folded or partially rolled-up condition.
- FIG. 9 shows the bag 10 containing, for example, 500 cc. whole blood received from a donor and introduced into the bag by way of its main inlet port 11. Since, as above mentioned, the volumetric capacity of the bag substantially exceeds the volume of the donor's blood collected therein, only the upper portion of the bag is filled with blood, as in FIG. 9, which quantity of blood may then be sealed in the bag as a solid pack by heat-sealing together the opposite side walls of the bag along the transverse line of the clamp 22 initially applied to the bag at the proper level for the volume of blood to be collected and contained in the bag. When a predetermined quantity of blood is collected in the bag, as shown in FIG. 9, and then sealed therein, it may be dispensed therefrom by way of any one of the ports which is in communication with the blood-containing portion of the bag.
- the interior of the bag as manufactured is effectively purged of air, the blood taken from a donor is readily introduced into the bag through the inlet port 11 without the need of providing the bag with any air venting means.
- the bag shape automatically adjusts itself accordingly as the volume of blood in the bag changes and thus there is never any danger of the blood being contaminated by contact with outside air.
- FIG. 12 shown the bag divided into two separately sealed compartments each containing a portion of the blood initially collected in the bag
- FIG. 13 shows the bag as it might be divided into three separately sealed compartments.
- This division of the blood quantity collected in the bag into two or more smaller quantity portions is readily effected by squeezing apart within the bag those portions of the blood which are to be separated from one another, then clamping together the intermediate juxtaposed sidewall portions of the bag by means of clamps 22-22 to provide therebetween an occluded area 23 and then heat-sealing together, as by a suitable electrically energized heat elements 24 extending transversely across the width of the bag, those portions of the bag side walls from between which the blood was expressed and occluded.
- the several compartments respectively containing separate sealed-in portions of the whole blood received from a single donor may then be cut apart, as at 25 (FIG. 12) or along the lines designated 25a (FIG.
- FIG. b and 7 illustrate a form of hemostatic clamp which may be employed for creating the above mentioned occluded areas to be heat-sealed together, which clamp as shown consists simply of a wire bent upon itself to provide a pair of arms 26-26 joined together by a spring loop 27 which normally biases the arms into open condition as shown in FIG. 6.
- the arms 26-26 are designed to straddle the bag width-wise and when pressed together squeeze the juxtaposed particular walls of the bag into such close contact as to prevent flow of the fluid in the bag past the line of contact between the bag side walls.
- the clamp is held in its locked position by a retainer clip 28 fixed to a free end of one arm 26 for removably receiving the free end of the other spring-pressed arm 26.
- any other suitable hemostatic clamp may be employed in lieu of that shown and described and accordingly it will be understood that the present invention is not limited or restricted to the use of the particular clamp as shown.
- FIG. 14 illustrates how the bag of the present invention may be further separated into several self-contained bloodfilled compartments.
- the bag instead of dividing the bag into vertically spaced units, it may be divided into several laterally spaced compartments 28 each of which may extend the full length of the bag or it may be divided, as shown, into several such laterally spaced compartments 28 of shorter length than the overall length of the bag formed in the upper portion thereof plus an additional compartment 29 formed in the bottom portion of the bag.
- all of the sealed-apart compartments may be cut apart as previously described, or any one of the sealed compartments may be cut apart from the bag as a whole, leaving intact the remaining portion of the bag with a plurality of bloodsealed compartments to be subsequently cut apart as may be desired or required for use.
- the intermediate T-connection I6 thereof is provided at its exit end with a removable closure, such as the ball-type seal 17, so that should one of the individually sealed compartments encompass the T-connection, severance of the tube at any point along its length would not result in discharge of the fluid contained in such sealed compartment except by way of a dispensing tube having a needle at one end projected through the stopper closing that particular one of the exit ports which is used.
- the several exit ports 12 which are suitably sealed into the marginal edges of the bag are so strategically spaced as to provide each separately sealed compartment of the bag with at least one of said ports for discharge of the fluid content of each said separate compartment.
- the bag as a whole is of sub stantially larger volumetric capacity than the volume of blood initially collected therein.
- the bag It may be clamped substantially midway of its length, as by the hemostatic clamp 22 shown and described or any other suitable clamp, to provide two separated compartmented segments Illa and 10b only one of which, e.g. that designated 10!), may be filled to full capacity, as see FIG. 17, with whole blood from the donor introduced by way of the main entrance port 11.
- the remaining unfilled segment 10a of the bag provides free space necessary for fractionating the whole blood into two or more of its components as will be presently described.
- This unfilled segment 10a which remains sealed and purged of air may be folded flatwise against one side wall of the filled segment of the bag, as shown in FIGS. 17 and 18, or it may be rolled up and secured compactly against the side wall of the filled segment of the bag by a strip of adhesive tape 31, as shown in FIG. 19.
- it may be held suspended from a suitable support either by its hanger elements 20-20 provided at the top end of the bag or by such elements fixed to the bag intermediate its opposite ends, as in the region of the clamp shown in FIG. 15 to 19.
- the precise location of the clamp 23 will depend upon the volume of whole blood initially collected therein, its position being such that the blood-expanded portion of the bag is completely filled with blood and so contains no free space into and from which the contained blood may shift.
- Such solid packing of the blood in the blood-containing portion 10b of the bag is a requisite for proper and effective centrifugation of the blood required for separating its several components.
- by providing such a solidly packed envelope of blood having no cumbersome appendages or connected auxiliary bags it becomes a simple matter to place and properly hold the envelope in a centrifuge for centrifugation of the whole blood.
- FIGS. 20 to 26 illustrate the procedure involved with use of the bag of the present invention for fractionation of the whole blood as originally collected from a donor into its several component parts, such as red cells, plasma, platelets and/or leukocytes.
- the whole blood collected from a donor is solidly packed in the portion llllb of the bag as shown in FIG. 17 and that the bag is conditioned as shown in FIG. 18 or in FIG, 19 with the hemostatic clamp 22 fixed in position
- the bag so filled with whole blood is placed in a centrifuge and therein subjected to such centrifugation as to separate the whole blood into its red cell and plasma parts.
- the centrifuging is conducted at a speed and for a period of time sufficient to effect isolation of the red cells and plasma within the portion 101) of the bag, without necessitating any reentry into the bag.
- the plasma component being of lower specific gravity than the red cells, will appear as a strata above the red cells and be separated from the latter by an intervening narrow strata of leukocytes,
- the plasma contains platelets which require for their isolation from the plasma, a second centrifugation at a speed andfor a time longer than that required for separating out the red cells from the whole blood.
- the centrifuged bag ll) is then carefully unfolded and suspended full length with its main entry tube presenting downwardly, following which the hemostatic clamp 22 is removed, so that as shown in FIG. 20 the suspended bag contains a bottom strata of red cells, an intermediate layer of leukocytes, a top strata of platelets-containing plasma and an upper section of free space, designated S.
- a hemostatic clamp 22a (see FIG. 21) is clamped upon the bag along a line demarcating the upper level of the red cells and the bag is then squeezed in the region immediately above said clamp to shift the fluid content away from the red cells to provide an occluded zone between the red cells and the leukocytes.
- a second clamp 22b is then clamped upon the bag along the upper line of this occluded zone to prevent reentry of the shifted fluid into said zone, whereupon, while both clamps remain fixed in position, the opposite walls of the bag in the occluded zone thereof are heat-sealed together, as at 32.
- This heat-sealed area 32 now serves effectively to seal and contain the red cells within a compartment 33 formed in the bottom of the bag.
- the two clamps 22a and 22b may then be removed from the bag.
- the same pair of clamps 22a and 22b may be successively applied to the bag, as shown in FIG. 22, to shift the plasma away from the layer of leukocytes which immediately overlies the heat-seal and to provide between the plasma and the leukocyte an occluded zone to be heat-sealed, as at 34.
- the leukocytes Upon completion of this heat-seal and removal of the clamps, the leukocytes will be sealed within a narrow compartment 35 disposed just above that containing the sealed-in red cells, as shown in FIG. 23.
- the plasma contained in the bag above the heat-seal 34 includes platelets and for isolation of these platelets from the plasma, a hemostatic clamp 22 is applied to the bag along the line 36 delineating the upper level of the plasma contained in the bag as shown in FIG. 23.
- This clamp 22c applied as shown in FIG. 24, solidly packs in the bag the plasma content thereof against shifting movement therein and with the plasma-containing platelets thus temporarily sealed between the heat-seal 34 and the clamp 220, the bag is subjected to a second centrifugation at the higher speed and longer time above mentioned for isolating the platelets from the plasma.
- the bag may again be folded upon itself so that the plasma-filled portion thereof is disposed in juxtaposed relation to the portion thereof containing the separately sealedin red cells and leukocytes.
- the bag is again suspended full length as shown in FIG. 24 in which condition the platelets will appear separated from the plasma along the line 37.
- the clamp 22c is removed and a pair of clamps 22a and 22b are again applied to the bag, as
- red cells, the leukocytes, the platelets and the plasma are isolated from one another and individually contained in the separately sealed compartments, respectively designated 33, 35, 39, and 40, which last-mentioned compartment containing the plasma is of substantially greater volume than the volume of plasma contained therein by reason of the fact that it includes the free space initially provided in the top portion of the bag.
- the isolated platelets end up in a sealed section of the bag located between the sealed red cell and leukocyte sections and the sealed plasma section. In some instances, however, it is found desirable to locate the sealed platelets at one end of the bag so that upon severing the platelet section from the bag, the remainder of the bag, containing the sealed red cell and plasma sections thereof, remains intact.
- FIGS. 27 to 30 are illustrative of this alternative procedure for fractionating the components of the whole blood collected in the bag.
- this alternate procedure following the first centrifugation above mentioned to isolate the red cells and leukocytes and after these components have been sealed in their respectively separate sections 33 and 35 of the bag as shown in FIG. 23, the bag is inverted from its position shown in said FIG. into its position as shown in FIG. 27.
- the compartments 33 and 35 in which the red cells and leukocytes are separately sealed, are located in the top portion of the bag, while the platelet-containing plasma drops to the bottom of the bag leaving a free space therein between the upper level of the plasma, indicated by the line 41, and the second or bottom heat-seal of the leukocyte section.
- a hemostat clamp 24d is then applied to the bag along the line 41 to compact the platelet containing plasma for centrifugation thereof, resulting in separation of the plasma and the platelets, which latter because of its greater specific gravity forms a strata below that of the plasma.
- the platelets are confined within their own sealed compartment 43 at the very bottom of the bag, while the plasma is confined within its own sealed compartment 44 intermediate the platelets and the sealed-in leukocytes.
- the intervening compartment 44 in which the plasma is sealed is of substantially greater volumetric capacity than the volume of the plasma contained therein, due again to the fact that the compartment 44 includes a good measure of the free space originally contained in the bag.
- each of the individually sealed compartments of the bag which respectively contain separated components of the whole blood originally collected in the bag, is provided with at least two ports, one of which may serve as an entrance port and the other as an exit port
- any one or more of the several compartments may be severed from the bag to provide a self-contained hermetically sealed unit containing a fractionated portion of the blood for such separate infusion or other use thereof as may be required or desired without necessitating any reentry of any of the sealed compartments.
- the provision of free space in at least one of the compartments is of importance.
- the red cells may be squeezed by way of the tube 13 into the plasma-containing section of the bag, to do which the portion of the tube extending below the T-connection 16 would necessarily be clamped closed and the ball valve 17 pushed out to provide communication only between the red cell and plasma-containing compartments.
- connecting tube 13 may be provided with additional T-connections beyond the single one shown to provide intercommunication between any two or more of the separated compartments and that other modifications or changes may be made from time to time without departing from the essential principles or real spirit of the present invention. It is accordingly intended to claim the invention broadly, as well as specifically, as indicated by the appended claims.
- Apparatus for the collection, treatment and storage of whole blood comprising a hermetically sealed, air evacuated, collapsible bag having a pair of perimetrally sealed juxtaposed side wall panels of substantially nonstretchable thermoplastic material, said side wall panels being collapsed against one another when the bag is in its empty condition, a plurality of ports communicating with the interior of the bag spaced from one another about the bag perimeter whereby upon division of the bag within the confines of its perimeter into a plurality of adjoining regions each such region is provided with at least one port for introduction and administration of that portion of the blood which is confined within a given region of the bag, each of said ports being sealed by needle-pierceable closure means, said bag being further characterized by the fact that its volumetric capacity is such that when a given supply of donor's blood is collected therein through any one of said entry ports a substantial amount of free space remains present in the bag between sidewall portions thereof having substantial areas of their inner faces in intimate flatwise contact, said free space being sufficient to permit shifting of portions of the blood
- Apparatus for the collection, treatment and storage of whole blood comprising a hermetically sealed, air-evacuated, collapsible bag having a pair of perimetrally sealed juxtaposed side wall panels of substantially nonstretchable thermoplastic material, said side wall panels being collapsed against one another when the bag is in its empty condition, a plurality of ports communicating with the interior of the bag spaced from one another about the bag perimeter whereby upon division of the bag within the confines of its perimeter into a plurality of adjoining regions each such region is provided with at least one port for introduction and administration of that portion of the blood which is confined within a given region of the bag, each of said ports being sealed by needle-pierceable closure means, said bag being further characterized by the fact that its volumetric capacity is such that when a given supply of donor's blood is collected therein a substantial amount of free space remains present in the bag sufficient to permit shifting of portions of the blood from one to another region of the bag by applying external pressure to the opposite sidewall panels of the bag and so create occlude
- conduit means is connected at its opposite ends to spaced regions of the bag and is provided intermediate its opposite ends with at least one connection to an intermediate region of the bag.
- conduit means is adapted to be selectively sealed against flow of fluent through its points of connection with the bag interior.
- At least one of said points of connection of the conduit with the bag interior includes a sealing element in said conduit which is shiftable by manipulation of the conduit wall from a position in which it normally seals said one point of connection against flow of fluent therethrough into a'position opening said point of connection to flow therethrough.
- conduit means may be sealed against flow therethrough by clamping the conduit tubing closed at any point along its length.
- Blood component separation apparatus comprising a single baglike receptacle formed of flexible, hemorepellant plastic sheet material, said receptacle being formed of a pair of juxtaposed wall panels having their perimetral edges sealed to provide therebetween an hermetically sealed, air-evacuated interfacial region adapted to receive and contain a measured quantity of blood collected from a donor, means associated with said receptacle and manipulable externally thereof for isolating within the receptacle separated components of the blood and respectively sealing the same in separate individual compartments formed in the single receptacle and means integral with said single receptacle and disposed externally of its interfacial region for effecting communication between certain of said individually sealed compartments of the receptacle for reconstituting the blood components respectively contained in said communicating compartments.
- said interfacial region is of a volumetric capacity when expanded substantially greater than the volume of blood collected in the receptacle whereby said volume of blood may be packed solidly against shifting within a portion of said interfacial region with the remainder of said region left in flattened condition in the form of a flap positionable against a side of the blood-filled portion of the receptacle for centrifugation of the collected blood.
- each of the individually sealed compartments containing an isolated blood component is provided with an exit port having a needlepierceable closure through which the isolated component may be withdrawn for use as desired.
- An aseptic single bag closed system for receiving, storing and separating a measured quantity of whole blood into several smaller quantities or fractionating said whole blood to isolate two or more components thereof, said system including a single bag formed of a pair ofjuxtaposed, flexible side wall panels of hemorepellant, flexible, plastic material perimetrally joined together to provide an air-evacuated interfacial region, a plurality of perimetrally spaced ports respectively affording access to different portions of said interfacial region, flexible fluid conduit means external of said interfacial region formed integrally with said bag for effecting communication between certain of said different portions of said interfacial region, and means associated with said bag and manipulable externally thereof for isolating and sealing within said single bag separate portions of the blood or components thereof.
Description
United States Patent Inventor Eugene D. Ross Elkins Park, Pennsylvania Appl. No. 616,005 Filed Feb. 14, 1967 Patented Dec. 8, 1970 Assignee Eugene Ross Laboratories, Inc.
Southampton, Pennsylvania a corporation of Pennsylvania APPARATUS FOR AND METHOD OF Primary ExaminerWilliam I. Price Attorney-Edelson and Udell ABSTRACT: An apparatus for and method of collecting and dividing whole blood into separated portions and fractionating the same into selected component parts thereof all within a single deformable bag formed of flexible plastic sheet material and providing a completely closed system requiring no reentry from outside of the bag for the purpose of dividing or fractionating the collected blood, separated portions of the whole blood and fractionated components thereof being self-contained in sealed apart sections of the bag which may be severed from the bag as individual self-contained sealed units each having at least one or more needle-pierceable ports for introducing or administering the bag content; the bag being further integrally provided with a side connecting tube affording communication between certain selected sealed sections of the bag for reconstituting the collected blood or a constitutent portion thereof as desired.
PATENTEU DEB 8197B sum 2 A Armwmo:
PATENTED DEC 81970 SHEET l 0F 4 PI IZ Red CcHS Plasma l/Vl/HVIUR EUGENE D- Ross fcfzQamfl d aw;
APPARATUS FOR AND METHOD OF COLLECTING, STORING, SEPARATING AND DISPENSING BLOOD AND BLOOD COMPONENTS DESCRIPTION OF THE INVENTION This invention relates to the handling and administering of blood and its components and more particularly to an improved apparatus for and method of collecting and storing whole blood, dividing an initially collected quantity of the blood into smaller discrete quantities, separating out such components of the collected whole blood as its red cells, plasma, platelets, leukocytes and the like for separate utilization thereof as may be desired, reconstituting the separated cells and plasma or other components of the blood, and otherwise handling the collected blood, including centrifugation thereof, all of which operations are performed in a single flexible envelop the interior of which is sealed from atmosphere and provides for fractionating the whole blood into separately usable smaller portions or components thereof completely free of any danger of air embolism and under such bacteriologically sterile and aseptic conditions as to practically eliminate any chance of contamination from external sources.
The sterile collecting and handling of human whole blood and the fractionation thereof into its component parts or into smaller portions has presented numerous serious problems which have not heretofore been adequately solved. Among these problems has been that of insuring against contamination and degradation of the blood and its separated components, such as cells, plasma, platelets and leukocytes by occlusion of and contact with air and other aseptically deleterious agents. Recognizing these problems, various attempts have been made in the past to solve them by providing closed systems consisting of a plurality of flexible plastic bags interconnected by tubing, by means of which whole blood or portions thereof collected in one bag might be squeezed into one or more other bags of the system without incurring any danger of contamination by infectious agents.
Since it is necessary for fractionation of the whole blood into its component parts to subject the blood to one or more centrifugations, the use of such plural bag closed systems has not been entirely satisfactory because of the difficulty and inconvenience encountered in fitting the several bags of the system and their appendages in the centrifuging apparatus, in addition to which the transfer of fluid from one bag to another has proved to be a cumbersome and inconvenient procedure because of the necessity of maintaining in some semblance of order the interconnecting tubing and other appendages which are incident to the multiple bag system. Further, the cost of such system is quite considerable, particularly when it is considered that in many instances the blood is not required to be separated into its components or is only required to be separated into cells and plasma, and that frequently the need for such separation is not known at that time the blood is initially collected, so that all of the bags of the system are not always required to be used and represent unnecessary cost and expense.
Having in mind the foregoing, it is a primary object of the may invention to provide a single bag closed system for collecting and handling whole blood and within which practically all of the operations capable of being performed in the plural bag systems now in use may be carried out efficiently, conveniently and with minimum danger of contamination of the collected blood or any fractionated portion or component part thereof.
A further object of the present invention is to provide a single bag closed system for collecting and handling blood which is compact and simple to set up and use, which may be packaged and sterilized at the source of its manufacture as a complete transfusion unit and which is relatively inexpensive to manufacture and economical to use.
Other objects and advantages of the invention will be apparent from the description which follows, it being understood that the present invention consists substantially in the combination, construction, location and relative arrangement of parts of the apparatus, as well as in its method as use, all as hereinafter described in detail, as shown in the accompanying drawings and as finally pointed out in the appended claims.
In the accompanying drawings:
FIG. I is a side elevational view of the closed system single receptacle unit constructed in accordance with and embodying the principles of the present invention, certain portions thereof being shown in section;
FIG. 2 is a detail sectional view as taken along the line 2-2 of FIG. 1;
FIG. 3 is a sectional view of the same detail as taken along the line 3-3 of FIG. 2;
FIG. 4 is a vertical sectional view of the receptacle as taken along the line 4-4 of FIG. 1;
FIG. 5 is a detail section view as taken along the line 5-5 of FIG. 1;
F IG. 6 is an elevational view of a clamp adapted to seal portions of the receptacle from one another;
FIG. 7 is a view showing the clamp of FIG. 6 in its closed clamping condition;
FIG. 8 is an end elevational view of the clamp as viewed from the line 8-8 of FIG. 7;
FIGS. 9 to 13 inclusive are vertical sectional views each taken along the line 4-4 of FIG. 1 showing the receptacle variously compartmentalized with each compartment filled with a portion of the initially collected blood;
FIG. 14 is an elevational view showing the receptacle of FIG. 1 horizontally and vertically separated into several blood-receiving compartments;
FIG. 15 is a perspective view showing the receptacle of FIG. 1 in partially folded condition with the clamp attached to provide two compartments separated along the fold line;
FIG. 16 is a vertical sectional view of the unit as taken along the line 16-16 ofFIG. 15;
FIG. 17 is a perspective view of the unit shown in FIG. 15 in its completely folded condition, showing the upper compartment filled with collected blood;
FIG. 18 is a vertical sectional view of the blood-filled unit as taken along the line 18-18 of FIG. 17;
FIG. 19 is a vertical sectional view corresponding to FIG. 18 but showing the unfilled portion of the receptacle in rolled-up condition instead of being folded flatwise against the filled portion as in FIG. 18;
FIG. 20 to 26 inclusive are elevational views illustrating the successive steps employed for fractionating within the receptacle the whole blood initially collected therein as shown in FIGS. 17 and 18 into its component parts of red cells, plasma, platelets and leukocytes; and
FIGS. 27 to 30 inclusive are elevational views illustrating the successive steps of an alternate procedure which may be employed for separating out the red cells, plasma and platelets of the whole blood initially collected and stored in the receptacle.
Referring now to the drawings and more particularly to FIGS. 1 to 5, it will be observed that the blood-handling ap paratus of the present invention essentially comprises a single receptacle or container in the form of a flexible-walled deformable bladder or bag 10 of generally rectangular outline having its perimetral edges completely sealed and fitted at preselected points with a main port 11 and a plurality of auxiliary ports 12 arranged in spaced relation to the main port and to one another about the marginal edge of the bag. The bag 10 may be formed of a single sheet of suitable plastic material which is folded upon itself to provide a pair of juxtaposed panels having their registering free edges heat-sealed together, or it may be formed of a pair of separate plastic sheets having all of their free edges suitably sealed together, as by heat-sealing, the use of adhesives or otherwise. The bag 10 is preferably formed of sheet material manufactured of polyvinyl, polypropylene, polyethylene fluorocarbon (e.g. Teflon and Kel-F) derivatives and like thermoplastic resins sufficiently transparent or translucent for visual inspection of the contents of the bag and capable of being heat-sealed together. The plastic material additionally should be of medical quality, flexible and sufficiently tough to withstand autoclaving, centrifugation and the extremes of heat and cold to which the filled bag may be subjected during treatment and storage of its contents.
A further essential characteristic of the bag of the present invention is that it be of a size and capacity substantially greater than that required for receiving and accommodating therein the quantity of whole blood initially collected from the donor. Thus, in a case where 500 cc. of whole blood received from the donor is collected in the bag, the volumetric capacity of the bag should desirable be from 750 to 1,000 cc. in order to effectually carry out the procedures in accordance with principles and objectives of the present invention.
In addition to the several ports 11 and 12 provided in the marginal edges of the bag, it is also provided along one of its longer sides with a flexible tube 13 having its opposite ends 14 and 15 in free communication with the interior of the bag and an intermediate portion connected, as by way of a T-connection 16, to the bag for communication with the bag interior. However, the inner end of the T-connection is normally closed by a removable ball-type seal 17 to prevent passage of material into and out of the bag by way of the T-connection 16 except under certain desired conditions, as will appear more fully hereinafter. This ball-type seal or closure member 17 is preferably in the form of a marble of glass, metal or nonporous other material inert to blood having a diameter somewhat greater than the internal diameter of the T-connection tubing so that when it is fitted in the inner end of said tubing the latter is expanded and holds the marble in its sealing position until such time that it is unseated from the tubing by pressure applied against the latter to squeeze the ball out of the tubing and into the cavity of the bag. It will be understood that any other suitable readily openable closure means may be provided in lieu of the ball closure 17 for maintaining the T-connection l6 closed against flow of fluid therethrough until such time as such flow may be desired.
In order to prevent undesired flow of fluid from one to another section of the bag through the side connecting tube 13, its opposite ends may also be fitted with displaceable closures, such as the ball closure 17. Alternatively, the tube 13 may be pinched closed adjacent any one of its points of entry into the bag by any suitable clamping means (not shown), which may be removed as required to effect communication by way of the tube between separately sealed portions of the bag.
The tube 13 with its T-connection 16 may be formed as an integral part of the bag 10, it being only necessary that this tube be substantially as flexible as the material of which the bag is formed. The main port 11 and the several auxiliary ports 12 are also formed of flexible tubing having their outer ends each closed by a stopper 19 having a core or diaphragm which may be readily pierced by the needle normally fitted on the tube employed for collecting or dispensing blood and its components. The auxiliary ports 12 may be employed in certain instances as inlet or entry ports and in other instances as exit or discharge ports and to this end these auxiliary ports are so perimetrally spaced about the bag as to provide at least one port, in each of the several separate zones or compartments into which the bag may be divided as hereinafter described so that each zone or compartment may thereby be provided with an inlet and/or outlet port.
Finally, it will be noted that the bag 10 is provided at each of its opposite ends and upon one of the sidewall panels thereof substantially midway between its opposite ends with a pair of laterally spaced hanger or support elements 20-20 by means of which the bag may be suspended from either end in its fully extended condition or from its middle in folded or partially rolled-up condition.
The bag 10 of the present invention may be employed, as illustrated in FIGS. 9 to 14 inclusive, for fractionating into smaller portions the volume of whole blood or other fluid initially collected in the bag. Thus, FIG. 9 shows the bag 10 containing, for example, 500 cc. whole blood received from a donor and introduced into the bag by way of its main inlet port 11. Since, as above mentioned, the volumetric capacity of the bag substantially exceeds the volume of the donor's blood collected therein, only the upper portion of the bag is filled with blood, as in FIG. 9, which quantity of blood may then be sealed in the bag as a solid pack by heat-sealing together the opposite side walls of the bag along the transverse line of the clamp 22 initially applied to the bag at the proper level for the volume of blood to be collected and contained in the bag. When a predetermined quantity of blood is collected in the bag, as shown in FIG. 9, and then sealed therein, it may be dispensed therefrom by way of any one of the ports which is in communication with the blood-containing portion of the bag.
Since the interior of the bag as manufactured is effectively purged of air, the blood taken from a donor is readily introduced into the bag through the inlet port 11 without the need of providing the bag with any air venting means. As the blood is collected in the bag or dispensed therefrom the bag shape automatically adjusts itself accordingly as the volume of blood in the bag changes and thus there is never any danger of the blood being contaminated by contact with outside air.
It is frequently desired that small amounts of the initially collected blood b made available for use, as where one or more infant-size infusions of whole blood are required each derived from a single adult donation of blood. The bag 10 of the present invention permits such fractionation of a single quantity of blood obtained from a donor. Thus, FIG. 12 shown the bag divided into two separately sealed compartments each containing a portion of the blood initially collected in the bag, while FIG. 13 shows the bag as it might be divided into three separately sealed compartments.
This division of the blood quantity collected in the bag into two or more smaller quantity portions is readily effected by squeezing apart within the bag those portions of the blood which are to be separated from one another, then clamping together the intermediate juxtaposed sidewall portions of the bag by means of clamps 22-22 to provide therebetween an occluded area 23 and then heat-sealing together, as by a suitable electrically energized heat elements 24 extending transversely across the width of the bag, those portions of the bag side walls from between which the blood was expressed and occluded. The several compartments respectively containing separate sealed-in portions of the whole blood received from a single donor may then be cut apart, as at 25 (FIG. 12) or along the lines designated 25a (FIG. 13), to provide individual self-contained blood-containing units each having one or more exit ports 12 through which the blood may be discharged either by gravity flow from th bag when suspended above the point of its application or by squeezing it out of the deformable bag unit. In certain instances instead of heat-sealing the occluded area 23, it may be maintained sealed against entry of fluid thereinto by the clamps applied permanently to the bag and suitably spaced apart between which clamps the bag may be cut apart.
FIG. b and 7 illustrate a form of hemostatic clamp which may be employed for creating the above mentioned occluded areas to be heat-sealed together, which clamp as shown consists simply of a wire bent upon itself to provide a pair of arms 26-26 joined together by a spring loop 27 which normally biases the arms into open condition as shown in FIG. 6. The arms 26-26 are designed to straddle the bag width-wise and when pressed together squeeze the juxtaposed particular walls of the bag into such close contact as to prevent flow of the fluid in the bag past the line of contact between the bag side walls. The clamp is held in its locked position by a retainer clip 28 fixed to a free end of one arm 26 for removably receiving the free end of the other spring-pressed arm 26. Of course, any other suitable hemostatic clamp may be employed in lieu of that shown and described and accordingly it will be understood that the present invention is not limited or restricted to the use of the particular clamp as shown.
FIG. 14 illustrates how the bag of the present invention may be further separated into several self-contained bloodfilled compartments. Thus, instead of dividing the bag into vertically spaced units, it may be divided into several laterally spaced compartments 28 each of which may extend the full length of the bag or it may be divided, as shown, into several such laterally spaced compartments 28 of shorter length than the overall length of the bag formed in the upper portion thereof plus an additional compartment 29 formed in the bottom portion of the bag. In this latter form of division of the bag, it may be desirable to first separate the bag into two main vertically spaced compartments and then after cutting the same apart, as at 30, thereafter separating the upper compartment into its several laterally spaced sections as shown. Of course, all of the sealed-apart compartments may be cut apart as previously described, or any one of the sealed compartments may be cut apart from the bag as a whole, leaving intact the remaining portion of the bag with a plurality of bloodsealed compartments to be subsequently cut apart as may be desired or required for use.
Of course, when any separately sealed compartments of the bag is severed therefrom, it will be necessary to also cut through the side connecting tube 13 and it is for this reason that the intermediate T-connection I6 thereof is provided at its exit end with a removable closure, such as the ball-type seal 17, so that should one of the individually sealed compartments encompass the T-connection, severance of the tube at any point along its length would not result in discharge of the fluid contained in such sealed compartment except by way of a dispensing tube having a needle at one end projected through the stopper closing that particular one of the exit ports which is used. As previously mentioned, the several exit ports 12 which are suitably sealed into the marginal edges of the bag are so strategically spaced as to provide each separately sealed compartment of the bag with at least one of said ports for discharge of the fluid content of each said separate compartment.
Also, as previously mentioned, the bag as a whole is of sub stantially larger volumetric capacity than the volume of blood initially collected therein. Thus, referring to FIGS. 15 to 18, it will be observed that the bag It) may be clamped substantially midway of its length, as by the hemostatic clamp 22 shown and described or any other suitable clamp, to provide two separated compartmented segments Illa and 10b only one of which, e.g. that designated 10!), may be filled to full capacity, as see FIG. 17, with whole blood from the donor introduced by way of the main entrance port 11. The remaining unfilled segment 10a of the bag provides free space necessary for fractionating the whole blood into two or more of its components as will be presently described.
This unfilled segment 10a, which remains sealed and purged of air may be folded flatwise against one side wall of the filled segment of the bag, as shown in FIGS. 17 and 18, or it may be rolled up and secured compactly against the side wall of the filled segment of the bag by a strip of adhesive tape 31, as shown in FIG. 19. In this partially filled condition of the bag, it may be held suspended from a suitable support either by its hanger elements 20-20 provided at the top end of the bag or by such elements fixed to the bag intermediate its opposite ends, as in the region of the clamp shown in FIG. 15 to 19.
Of course, the precise location of the clamp 23 will depend upon the volume of whole blood initially collected therein, its position being such that the blood-expanded portion of the bag is completely filled with blood and so contains no free space into and from which the contained blood may shift. Such solid packing of the blood in the blood-containing portion 10b of the bag is a requisite for proper and effective centrifugation of the blood required for separating its several components. Also, by providing such a solidly packed envelope of blood having no cumbersome appendages or connected auxiliary bags, it becomes a simple matter to place and properly hold the envelope in a centrifuge for centrifugation of the whole blood.
FIGS. 20 to 26 illustrate the procedure involved with use of the bag of the present invention for fractionation of the whole blood as originally collected from a donor into its several component parts, such as red cells, plasma, platelets and/or leukocytes. Assuming that the whole blood collected from a donor is solidly packed in the portion llllb of the bag as shown in FIG. 17 and that the bag is conditioned as shown in FIG. 18 or in FIG, 19 with the hemostatic clamp 22 fixed in position, the bag so filled with whole blood is placed in a centrifuge and therein subjected to such centrifugation as to separate the whole blood into its red cell and plasma parts. The centrifuging is conducted at a speed and for a period of time sufficient to effect isolation of the red cells and plasma within the portion 101) of the bag, without necessitating any reentry into the bag. Upon completion ofsuch centrifugation, the plasma component, being of lower specific gravity than the red cells, will appear as a strata above the red cells and be separated from the latter by an intervening narrow strata of leukocytes, At this stage of the procedure, the plasma contains platelets which require for their isolation from the plasma, a second centrifugation at a speed andfor a time longer than that required for separating out the red cells from the whole blood.
The centrifuged bag ll) is then carefully unfolded and suspended full length with its main entry tube presenting downwardly, following which the hemostatic clamp 22 is removed, so that as shown in FIG. 20 the suspended bag contains a bottom strata of red cells, an intermediate layer of leukocytes, a top strata of platelets-containing plasma and an upper section of free space, designated S.
Thereupon, a hemostatic clamp 22a (see FIG. 21) is clamped upon the bag along a line demarcating the upper level of the red cells and the bag is then squeezed in the region immediately above said clamp to shift the fluid content away from the red cells to provide an occluded zone between the red cells and the leukocytes. A second clamp 22b is then clamped upon the bag along the upper line of this occluded zone to prevent reentry of the shifted fluid into said zone, whereupon, while both clamps remain fixed in position, the opposite walls of the bag in the occluded zone thereof are heat-sealed together, as at 32. This heat-sealed area 32 now serves effectively to seal and contain the red cells within a compartment 33 formed in the bottom of the bag. The two clamps 22a and 22b may then be removed from the bag.
Thereafter, the same pair of clamps 22a and 22b may be successively applied to the bag, as shown in FIG. 22, to shift the plasma away from the layer of leukocytes which immediately overlies the heat-seal and to provide between the plasma and the leukocyte an occluded zone to be heat-sealed, as at 34. Upon completion of this heat-seal and removal of the clamps, the leukocytes will be sealed within a narrow compartment 35 disposed just above that containing the sealed-in red cells, as shown in FIG. 23.
As above mentioned, the plasma contained in the bag above the heat-seal 34 includes platelets and for isolation of these platelets from the plasma, a hemostatic clamp 22 is applied to the bag along the line 36 delineating the upper level of the plasma contained in the bag as shown in FIG. 23. This clamp 22c, applied as shown in FIG. 24, solidly packs in the bag the plasma content thereof against shifting movement therein and with the plasma-containing platelets thus temporarily sealed between the heat-seal 34 and the clamp 220, the bag is subjected to a second centrifugation at the higher speed and longer time above mentioned for isolating the platelets from the plasma. For this second centrifuging operation, the bag may again be folded upon itself so that the plasma-filled portion thereof is disposed in juxtaposed relation to the portion thereof containing the separately sealedin red cells and leukocytes.
Upon completion of this second centrifugation, the bag is again suspended full length as shown in FIG. 24 in which condition the platelets will appear separated from the plasma along the line 37. Thereupon, the clamp 22c is removed and a pair of clamps 22a and 22b are again applied to the bag, as
shown in FIG. 25, to shift the plasma away from the platelets which now immediately overlay the heat-seal 34 and to provide between the plasma and the platelets an occluded zone to be heat-sealed, as at 38, and so provide a separately sealed compartment 39 for the platelets and a sealed compartment 40 for the plasma. Following completion of this last-mentioned heat-seal, the hemostatic clamps are removed and the bag 10 is then in its final condition as shown in FIG. 26 wherein the red cells, the leukocytes, the platelets and the plasma are isolated from one another and individually contained in the separately sealed compartments, respectively designated 33, 35, 39, and 40, which last-mentioned compartment containing the plasma is of substantially greater volume than the volume of plasma contained therein by reason of the fact that it includes the free space initially provided in the top portion of the bag.
It will not noted that in carrying out the procedure just described and as illustrated in FIGS. to 26, the isolated platelets end up in a sealed section of the bag located between the sealed red cell and leukocyte sections and the sealed plasma section. In some instances, however, it is found desirable to locate the sealed platelets at one end of the bag so that upon severing the platelet section from the bag, the remainder of the bag, containing the sealed red cell and plasma sections thereof, remains intact.
FIGS. 27 to 30 are illustrative of this alternative procedure for fractionating the components of the whole blood collected in the bag. In this alternate procedure, following the first centrifugation above mentioned to isolate the red cells and leukocytes and after these components have been sealed in their respectively separate sections 33 and 35 of the bag as shown in FIG. 23, the bag is inverted from its position shown in said FIG. into its position as shown in FIG. 27. In this latter posi' tion the compartments 33 and 35, in which the red cells and leukocytes are separately sealed, are located in the top portion of the bag, while the platelet-containing plasma drops to the bottom of the bag leaving a free space therein between the upper level of the plasma, indicated by the line 41, and the second or bottom heat-seal of the leukocyte section.
A hemostat clamp 24d is then applied to the bag along the line 41 to compact the platelet containing plasma for centrifugation thereof, resulting in separation of the plasma and the platelets, which latter because of its greater specific gravity forms a strata below that of the plasma. Upon then successively applying a pair of the hemostatic clamps as above described to shift the plasma in separated relation to the platelets, and then seal these components apart, as at 42, the platelets are confined within their own sealed compartment 43 at the very bottom of the bag, while the plasma is confined within its own sealed compartment 44 intermediate the platelets and the sealed-in leukocytes. As in the first procedure illustrated by FIGS. 20 to 26, the intervening compartment 44 in which the plasma is sealed is of substantially greater volumetric capacity than the volume of the plasma contained therein, due again to the fact that the compartment 44 includes a good measure of the free space originally contained in the bag.
It will be noted that each of the individually sealed compartments of the bag, which respectively contain separated components of the whole blood originally collected in the bag, is provided with at least two ports, one of which may serve as an entrance port and the other as an exit port Also any one or more of the several compartments may be severed from the bag to provide a self-contained hermetically sealed unit containing a fractionated portion of the blood for such separate infusion or other use thereof as may be required or desired without necessitating any reentry of any of the sealed compartments. It is in this connection that the provision of free space in at least one of the compartments is of importance. Thus, should it be desired to reconstitute, i.e., admix, the separately sealed in plasma and red cells in the bag of FIG. 26, this may be accomplished readily and without danger of contamination by contact with air or of creating any air embolism when the reconstituted fluid is given to the patient by the simple expedient of squeezing the red cells out of its sealed compartment into the plasma-containing compartment by way of the tube 13 the opposite ends of which are in sealed communication with the interiors of these two compartments.
If such reconstitution is desired in the bag prepared as in FIG. 30, the red cells may be squeezed by way of the tube 13 into the plasma-containing section of the bag, to do which the portion of the tube extending below the T-connection 16 would necessarily be clamped closed and the ball valve 17 pushed out to provide communication only between the red cell and plasma-containing compartments.
Should it be desired to reconstitute the plasma with platelets, this would be effected by use of the bag prepared as shown in FIG. 30 wherein the platelet-containing compartment is in communication with the plasma-containing compartment by way of the tube 13.
It will be understood, of course, that the connecting tube 13 may be provided with additional T-connections beyond the single one shown to provide intercommunication between any two or more of the separated compartments and that other modifications or changes may be made from time to time without departing from the essential principles or real spirit of the present invention. It is accordingly intended to claim the invention broadly, as well as specifically, as indicated by the appended claims.
Iclaim:
1. Apparatus for the collection, treatment and storage of whole blood comprising a hermetically sealed, air evacuated, collapsible bag having a pair of perimetrally sealed juxtaposed side wall panels of substantially nonstretchable thermoplastic material, said side wall panels being collapsed against one another when the bag is in its empty condition, a plurality of ports communicating with the interior of the bag spaced from one another about the bag perimeter whereby upon division of the bag within the confines of its perimeter into a plurality of adjoining regions each such region is provided with at least one port for introduction and administration of that portion of the blood which is confined within a given region of the bag, each of said ports being sealed by needle-pierceable closure means, said bag being further characterized by the fact that its volumetric capacity is such that when a given supply of donor's blood is collected therein through any one of said entry ports a substantial amount of free space remains present in the bag between sidewall portions thereof having substantial areas of their inner faces in intimate flatwise contact, said free space being sufficient to permit shifting of portions of the blood from one to another region of the bag by applying external pressure to the opposite side wall panels of the bag and so create occluded areas between said adjoining regions which may be hermetically sealed to compartmentalize the bag, and means for sealing the filled portion 2. An apparatus as defined in claim 1 wherein the free space portion of the bag, as a flattened end extension thereof, is foldable about the line of said sealing means flatwise against a sidewall of the filled portion of the bag.
3. An apparatus as defined in claim 1 wherein said flattened end extension of the bag is adapted to be rolled up and temporarily secured in its rolled-up condition against a sidewall of the filled portion of the bag.
4. An apparatus as defined in claim 1 wherein said occluded areas are heat-sealable to divide the bag into a plurality of separately sealed compartments each filled with a portion of the donors blood initially collected in the bag.
5. An apparatus as defined in claim 4 wherein said heatsealable occluded areas extend from side to side of the bag to provide a plurality of vertically spaced compartments.
6. An apparatus as defined in claim 4 wherein certain of said heat-scalable occluded areas extend longitudinally of the bag to provide the same with a plurality of laterally spaced compartments.
7. Apparatus for the collection, treatment and storage of whole blood comprising a hermetically sealed, air-evacuated, collapsible bag having a pair of perimetrally sealed juxtaposed side wall panels of substantially nonstretchable thermoplastic material, said side wall panels being collapsed against one another when the bag is in its empty condition, a plurality of ports communicating with the interior of the bag spaced from one another about the bag perimeter whereby upon division of the bag within the confines of its perimeter into a plurality of adjoining regions each such region is provided with at least one port for introduction and administration of that portion of the blood which is confined within a given region of the bag, each of said ports being sealed by needle-pierceable closure means, said bag being further characterized by the fact that its volumetric capacity is such that when a given supply of donor's blood is collected therein a substantial amount of free space remains present in the bag sufficient to permit shifting of portions of the blood from one to another region of the bag by applying external pressure to the opposite sidewall panels of the bag and so create occluded areas between said adjoining regions which may be hermetically sealed to compartmentalize the bag, said bag including as an integral part thereof flexible fluid-flow conduit means disposed in external relation to the bag interior and extending along at least one side edge of the bag to effect intercommunication between selected spaced regions of the bag interior.
8. An apparatus as defined in claim 7 wherein said conduit means is connected at its opposite ends to spaced regions of the bag and is provided intermediate its opposite ends with at least one connection to an intermediate region of the bag.
9. An apparatus as defined in claim 7 wherein said conduit means is adapted to be selectively sealed against flow of fluent through its points of connection with the bag interior.
It). An apparatus as defined in claim 9 wherein at least one of said points of connection of the conduit with the bag interior includes a sealing element in said conduit which is shiftable by manipulation of the conduit wall from a position in which it normally seals said one point of connection against flow of fluent therethrough into a'position opening said point of connection to flow therethrough.
11. An apparatus as defined in claim 9 wherein said conduit means may be sealed against flow therethrough by clamping the conduit tubing closed at any point along its length.
12. Blood component separation apparatus comprising a single baglike receptacle formed of flexible, hemorepellant plastic sheet material, said receptacle being formed of a pair of juxtaposed wall panels having their perimetral edges sealed to provide therebetween an hermetically sealed, air-evacuated interfacial region adapted to receive and contain a measured quantity of blood collected from a donor, means associated with said receptacle and manipulable externally thereof for isolating within the receptacle separated components of the blood and respectively sealing the same in separate individual compartments formed in the single receptacle and means integral with said single receptacle and disposed externally of its interfacial region for effecting communication between certain of said individually sealed compartments of the receptacle for reconstituting the blood components respectively contained in said communicating compartments.
13. Apparatus as defined in claim 12 wherein said interfacial region is of a volumetric capacity when expanded substantially greater than the volume of blood collected in the receptacle whereby said volume of blood may be packed solidly against shifting within a portion of said interfacial region with the remainder of said region left in flattened condition in the form of a flap positionable against a side of the blood-filled portion of the receptacle for centrifugation of the collected blood.
14. Apparatus as defined in claim 12 wherein each of the individually sealed compartments containing an isolated blood component is provided with an exit port having a needlepierceable closure through which the isolated component may be withdrawn for use as desired.
15. An aseptic single bag closed system for receiving, storing and separating a measured quantity of whole blood into several smaller quantities or fractionating said whole blood to isolate two or more components thereof, said system including a single bag formed of a pair ofjuxtaposed, flexible side wall panels of hemorepellant, flexible, plastic material perimetrally joined together to provide an air-evacuated interfacial region, a plurality of perimetrally spaced ports respectively affording access to different portions of said interfacial region, flexible fluid conduit means external of said interfacial region formed integrally with said bag for effecting communication between certain of said different portions of said interfacial region, and means associated with said bag and manipulable externally thereof for isolating and sealing within said single bag separate portions of the blood or components thereof.
16. An aseptic system as defined in claim 15 wherein said isolated sealed portions of the blood or its components are each severable from the bag as self-contained units respectively adapted to store and dispense as needed the fluent contained therein.
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US61600567A | 1967-02-14 | 1967-02-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US3545671A true US3545671A (en) | 1970-12-08 |
Family
ID=24467668
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US616005A Expired - Lifetime US3545671A (en) | 1967-02-14 | 1967-02-14 | Apparatus for and method of collecting,storing,separating and dispensing blood and blood components |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US3545671A (en) |
Cited By (97)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2257979A1 (en) * | 1971-11-26 | 1973-06-14 | Commissariat Energie Atomique | BLOOD COLLECTION AND TREATMENT DEVICE AND PROCEDURES FOR YOUR OPERATION |
| US3866608A (en) * | 1973-10-23 | 1975-02-18 | Sorenson Research Co | Aseptic suction collection system and method |
| US3888236A (en) * | 1972-03-28 | 1975-06-10 | Gunter Marx | Apparatus for measuring a quanity of urine |
| US3905477A (en) * | 1972-03-14 | 1975-09-16 | Union Carbide Corp | Tamperproof pouch label |
| US3911918A (en) * | 1972-04-13 | 1975-10-14 | Ralph D Turner | Blood collection, storage and administering bag |
| FR2290180A1 (en) * | 1974-11-06 | 1976-06-04 | Vidal Paul | Serum collection equipment - comprises closed plastic pouch with probe and conduit and means for hooking in place |
| US3965889A (en) * | 1971-11-26 | 1976-06-29 | Commissariat A L'energie Atomique | Apparatus for the sampling of blood and the separation of plasma under anaerobic conditions |
| US3987961A (en) * | 1974-01-29 | 1976-10-26 | Heraeus-Christ Gmbh | Centrifuge bag for treatment of biological liquids |
| US4026669A (en) * | 1975-07-14 | 1977-05-31 | Baxter Laboratories, Inc. | Variable capacity reservoir assembly |
| US4040959A (en) * | 1976-06-22 | 1977-08-09 | Berman Irwin R | Multi-purpose blood bag |
| US4059108A (en) * | 1974-08-15 | 1977-11-22 | Haemonetics Corporation | Process for pheresis procedure and disposable pheresis bowl therefor |
| US4098456A (en) * | 1977-03-29 | 1978-07-04 | Baxter Travenol Laboratories, Inc. | Centrifuge system having collapsible centrifuge bags |
| US4131200A (en) * | 1976-07-06 | 1978-12-26 | Union Carbide Corporation | Thermoplastic blood bag |
| US4136678A (en) * | 1976-08-31 | 1979-01-30 | Janet Beach | Method of admitting solutions to medical drainage or irrigation conduits |
| US4253458A (en) * | 1979-03-08 | 1981-03-03 | Baxter Travenol Laboratories, Inc. | Method and apparatus for collecting blood plasma |
| US4301963A (en) * | 1978-06-05 | 1981-11-24 | Beckman Instruments, Inc. | Integral one piece centrifuge tube |
| US4340049A (en) * | 1979-10-18 | 1982-07-20 | Baxter Travenol Laboratories, Inc. | Breakaway valve |
| US4386622A (en) * | 1979-10-18 | 1983-06-07 | Baxter Travenol Laboratories, Inc. | Breakaway valve |
| WO1984002091A1 (en) * | 1982-11-26 | 1984-06-07 | Seroteknik Hb | Method and apparatus for centrifugal batch separation of blood |
| WO1985004636A1 (en) * | 1984-04-12 | 1985-10-24 | Baxter Travenol Laboratories, Inc. | Disposable container having semi-rigid access end plug |
| EP0161551A2 (en) * | 1984-05-14 | 1985-11-21 | NPBI Nederlands Produktielaboratorium voor Bloedtransfusieapparatuur en Infusievloeistoffen B.V. | Apparatus for separating the blood plasma and the buffycoat from the blood in a flexible vessel |
| US4588554A (en) * | 1982-02-25 | 1986-05-13 | Fluilogic Systems Oy | Reagent package |
| US4610781A (en) * | 1983-12-30 | 1986-09-09 | Baxter Travenol Laboratories, Inc. | Fluid processing system with flow control manifold |
| US4649028A (en) * | 1985-03-27 | 1987-03-10 | Medica Corporation | Electrolyte analyzer |
| US4675010A (en) * | 1985-10-04 | 1987-06-23 | American Omni Medical, Inc. | Thoracic drainage collection system and method |
| US4705668A (en) * | 1985-03-27 | 1987-11-10 | Medica Corporation | Electrolyte analyzer |
| DE8716004U1 (en) * | 1987-12-03 | 1988-02-11 | Korth, Ruth, Dr.Med., 8000 Muenchen, De | |
| US4731053A (en) * | 1986-12-23 | 1988-03-15 | Merck & Co., Inc. | Container device for separately storing and mixing two ingredients |
| US4804363A (en) * | 1986-07-16 | 1989-02-14 | Autologous Blood Corporation | Apparatus and method for storing and processing blood |
| US4820297A (en) * | 1986-12-12 | 1989-04-11 | Baxter International Inc. | Fluid delivery system with integrally formed sample cell |
| US4900321A (en) * | 1986-12-12 | 1990-02-13 | Baxter International Inc. | Set with integrally formed sample cell |
| US4915847A (en) * | 1987-08-04 | 1990-04-10 | Baxter International Inc. | Cryoglobulin separation |
| US4917804A (en) * | 1986-10-31 | 1990-04-17 | Baxter International Inc. | Method and vessel for separation of cryoglobin |
| US4997083A (en) * | 1987-05-29 | 1991-03-05 | Vifor S.A. | Container intended for the separate storage of active compositions and for their subsequent mixing |
| US5030215A (en) * | 1990-01-03 | 1991-07-09 | Cryolife, Inc. | Preparation of fibrinogen/factor XIII precipitate |
| EP0438703A1 (en) * | 1989-12-29 | 1991-07-31 | Margrit Dipl.-Ing. Werner | System for the collection and retransfusion of autologous blood |
| USRE33924E (en) * | 1986-07-16 | 1992-05-12 | Autologous Blood Corp. | Apparatus and method for storing and processing blood |
| US5207509A (en) * | 1991-03-07 | 1993-05-04 | Fresenius Ag | Multichamber bag |
| WO1993021892A1 (en) * | 1992-04-23 | 1993-11-11 | Seroteknik Hb | System and method for centrifugal separation of two or more fractions from a composite liquid, and a bag intended therefor |
| EP0608882A1 (en) * | 1993-01-29 | 1994-08-03 | Terumo Kabushiki Kaisha | Apparatus for separation of liquid |
| US5656154A (en) * | 1995-06-07 | 1997-08-12 | Organ, Inc. | Method and apparatus for separating a fluid into components and for washing a material |
| WO1997042897A1 (en) * | 1996-05-13 | 1997-11-20 | B. Braun Medical | Flexible, multiple-compartment drug container and method of making and using same |
| WO1997049959A3 (en) * | 1996-06-25 | 1998-03-19 | Thermogenesis Corp | Freezing and thawing bag, mold, apparatus and method |
| US5910138A (en) * | 1996-05-13 | 1999-06-08 | B. Braun Medical, Inc. | Flexible medical container with selectively enlargeable compartments and method for making same |
| US5928213A (en) * | 1996-05-13 | 1999-07-27 | B. Braun Medical, Inc. | Flexible multiple compartment medical container with preferentially rupturable seals |
| US5944709A (en) * | 1996-05-13 | 1999-08-31 | B. Braun Medical, Inc. | Flexible, multiple-compartment drug container and method of making and using same |
| US6039720A (en) * | 1995-08-08 | 2000-03-21 | Gambro Ab | Bag for containing a sterile medical solution |
| US6039719A (en) * | 1995-08-08 | 2000-03-21 | Gambro Ab | Bag for containing a sterile medical solution and method of mixing a sterile medical solution |
| US6113575A (en) * | 1998-05-14 | 2000-09-05 | Terumo Cardiovascular Systems Corporation | Volume control apparatus for a flexible venous reservoir |
| US6183460B1 (en) * | 1998-01-22 | 2001-02-06 | Baxter International Inc. | Multi-use solution container having flaps |
| US6491678B1 (en) * | 1994-12-05 | 2002-12-10 | New York Blood Center, Inc. | Freezer bag |
| US20040045842A1 (en) * | 1998-05-29 | 2004-03-11 | Naoto Matsuda | Pouch having a branched chamber |
| US6808675B1 (en) | 1996-06-25 | 2004-10-26 | Thermogenesis Corp. | Freezing and thawing bag, mold, apparatus and method |
| US7169547B2 (en) | 1994-12-05 | 2007-01-30 | New York Blood Center, Inc. | High concentration white blood cells as a therapeutic product |
| US20070075016A1 (en) * | 2005-08-23 | 2007-04-05 | Biomet Manufacturing Corp. | Method and apparatus for collecting biological materials |
| US20070208321A1 (en) * | 2005-08-23 | 2007-09-06 | Biomet Manufacturing Corp. | Method And Apparatus For Collecting Biological Materials |
| US7374678B2 (en) | 2002-05-24 | 2008-05-20 | Biomet Biologics, Inc. | Apparatus and method for separating and concentrating fluids containing multiple components |
| US7470371B2 (en) | 2002-05-03 | 2008-12-30 | Hanuman Llc | Methods and apparatus for isolating platelets from blood |
| US20100047403A1 (en) * | 2006-10-30 | 2010-02-25 | Elizabeth Johnson | Pouch container for food product |
| US20100198185A1 (en) * | 2007-06-21 | 2010-08-05 | Goerisch Irmgard | Multi-chamber bag for use with enteral nutrition |
| US7780860B2 (en) | 2002-05-24 | 2010-08-24 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US7806276B2 (en) | 2007-04-12 | 2010-10-05 | Hanuman, Llc | Buoy suspension fractionation system |
| WO2010122542A1 (en) * | 2009-04-20 | 2010-10-28 | Fuil Technologies Limited | A container for storing a flowable bodily material, and a method for storing a flowable bodily material |
| US7832566B2 (en) | 2002-05-24 | 2010-11-16 | Biomet Biologics, Llc | Method and apparatus for separating and concentrating a component from a multi-component material including macroparticles |
| US7845499B2 (en) | 2002-05-24 | 2010-12-07 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US20110022022A1 (en) * | 2007-07-19 | 2011-01-27 | Tatsuro Tsuruoka | Multi-chamber bag |
| US20110143968A1 (en) * | 1998-06-24 | 2011-06-16 | Iquum, Inc. | Sample vessels |
| US7992725B2 (en) | 2002-05-03 | 2011-08-09 | Biomet Biologics, Llc | Buoy suspension fractionation system |
| US20110274849A1 (en) * | 2009-01-23 | 2011-11-10 | Maarten Van Pul | Packaging for two or more fluids |
| US8313954B2 (en) | 2009-04-03 | 2012-11-20 | Biomet Biologics, Llc | All-in-one means of separating blood components |
| US8328024B2 (en) | 2007-04-12 | 2012-12-11 | Hanuman, Llc | Buoy suspension fractionation system |
| US8337711B2 (en) | 2008-02-29 | 2012-12-25 | Biomet Biologics, Llc | System and process for separating a material |
| US8567609B2 (en) | 2006-05-25 | 2013-10-29 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US8591391B2 (en) | 2010-04-12 | 2013-11-26 | Biomet Biologics, Llc | Method and apparatus for separating a material |
| US8783470B2 (en) | 2009-03-06 | 2014-07-22 | Biomet Biologics, Llc | Method and apparatus for producing autologous thrombin |
| US9011800B2 (en) | 2009-07-16 | 2015-04-21 | Biomet Biologics, Llc | Method and apparatus for separating biological materials |
| US20150374583A1 (en) * | 2014-06-26 | 2015-12-31 | Advanced Scientifics, Inc. | Freezer bag, storage system, and method of freezing |
| US20160000652A1 (en) * | 2014-02-07 | 2016-01-07 | Eurozyto Gmbh | Container and kit for providing parenteral nutrition |
| US20160038275A1 (en) * | 2009-04-29 | 2016-02-11 | Keller Medical, Inc. | Silicone breast implant delivery |
| US20160038946A1 (en) * | 2014-08-11 | 2016-02-11 | Auxocell Laboratories, Inc. | Centrifuge clip and method |
| US9556243B2 (en) | 2013-03-15 | 2017-01-31 | Biomet Biologies, LLC | Methods for making cytokine compositions from tissues using non-centrifugal methods |
| US9642956B2 (en) | 2012-08-27 | 2017-05-09 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US9701728B2 (en) | 2008-02-27 | 2017-07-11 | Biomet Biologics, Llc | Methods and compositions for delivering interleukin-1 receptor antagonist |
| US9895418B2 (en) | 2013-03-15 | 2018-02-20 | Biomet Biologics, Llc | Treatment of peripheral vascular disease using protein solutions |
| US9897589B2 (en) | 2002-05-24 | 2018-02-20 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US9950035B2 (en) | 2013-03-15 | 2018-04-24 | Biomet Biologics, Llc | Methods and non-immunogenic compositions for treating inflammatory disorders |
| US10143725B2 (en) | 2013-03-15 | 2018-12-04 | Biomet Biologics, Llc | Treatment of pain using protein solutions |
| US10213294B2 (en) | 2007-12-07 | 2019-02-26 | Keller Medical, Inc. | Apparatus for use in a surgical procedure |
| US10328404B2 (en) | 2005-04-22 | 2019-06-25 | Life Technologies Corporation | Gas spargers and related container systems |
| US10350554B2 (en) | 2011-09-30 | 2019-07-16 | Life Technologies Corporation | Container with film Sparger |
| US10576130B2 (en) | 2013-03-15 | 2020-03-03 | Biomet Manufacturing, Llc | Treatment of collagen defects using protein solutions |
| US10589197B2 (en) | 2016-12-01 | 2020-03-17 | Life Technologies Corporation | Microcarrier filter bag assemblies and methods of use |
| CN111617887A (en) * | 2019-02-28 | 2020-09-04 | 赛默电子Led有限公司 | Extruder and extruder system for separating components of biological suspensions and methods of use |
| US20200306421A1 (en) * | 2019-04-01 | 2020-10-01 | Sterigear, Llc | Dual drainage bag, assemblies, and related methods |
| US10934514B2 (en) | 2011-09-29 | 2021-03-02 | Life Technologies Corporation | Filter systems for separating microcarriers from cell culture solutions |
| US20210187495A1 (en) * | 2017-06-22 | 2021-06-24 | Texas Heart Institute | Multi-compartment biological fluid storage device |
| US11957733B2 (en) | 2019-10-28 | 2024-04-16 | Biomet Manufacturing, Llc | Treatment of collagen defects using protein solutions |
-
1967
- 1967-02-14 US US616005A patent/US3545671A/en not_active Expired - Lifetime
Cited By (164)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2257979A1 (en) * | 1971-11-26 | 1973-06-14 | Commissariat Energie Atomique | BLOOD COLLECTION AND TREATMENT DEVICE AND PROCEDURES FOR YOUR OPERATION |
| US3965889A (en) * | 1971-11-26 | 1976-06-29 | Commissariat A L'energie Atomique | Apparatus for the sampling of blood and the separation of plasma under anaerobic conditions |
| US3905477A (en) * | 1972-03-14 | 1975-09-16 | Union Carbide Corp | Tamperproof pouch label |
| US3888236A (en) * | 1972-03-28 | 1975-06-10 | Gunter Marx | Apparatus for measuring a quanity of urine |
| US3911918A (en) * | 1972-04-13 | 1975-10-14 | Ralph D Turner | Blood collection, storage and administering bag |
| US3866608A (en) * | 1973-10-23 | 1975-02-18 | Sorenson Research Co | Aseptic suction collection system and method |
| US3987961A (en) * | 1974-01-29 | 1976-10-26 | Heraeus-Christ Gmbh | Centrifuge bag for treatment of biological liquids |
| US4059108A (en) * | 1974-08-15 | 1977-11-22 | Haemonetics Corporation | Process for pheresis procedure and disposable pheresis bowl therefor |
| FR2290180A1 (en) * | 1974-11-06 | 1976-06-04 | Vidal Paul | Serum collection equipment - comprises closed plastic pouch with probe and conduit and means for hooking in place |
| US4026669A (en) * | 1975-07-14 | 1977-05-31 | Baxter Laboratories, Inc. | Variable capacity reservoir assembly |
| US4040959A (en) * | 1976-06-22 | 1977-08-09 | Berman Irwin R | Multi-purpose blood bag |
| US4131200A (en) * | 1976-07-06 | 1978-12-26 | Union Carbide Corporation | Thermoplastic blood bag |
| US4136678A (en) * | 1976-08-31 | 1979-01-30 | Janet Beach | Method of admitting solutions to medical drainage or irrigation conduits |
| US4098456A (en) * | 1977-03-29 | 1978-07-04 | Baxter Travenol Laboratories, Inc. | Centrifuge system having collapsible centrifuge bags |
| US4301963A (en) * | 1978-06-05 | 1981-11-24 | Beckman Instruments, Inc. | Integral one piece centrifuge tube |
| US4253458A (en) * | 1979-03-08 | 1981-03-03 | Baxter Travenol Laboratories, Inc. | Method and apparatus for collecting blood plasma |
| US4340049A (en) * | 1979-10-18 | 1982-07-20 | Baxter Travenol Laboratories, Inc. | Breakaway valve |
| US4386622A (en) * | 1979-10-18 | 1983-06-07 | Baxter Travenol Laboratories, Inc. | Breakaway valve |
| US4588554A (en) * | 1982-02-25 | 1986-05-13 | Fluilogic Systems Oy | Reagent package |
| WO1984002091A1 (en) * | 1982-11-26 | 1984-06-07 | Seroteknik Hb | Method and apparatus for centrifugal batch separation of blood |
| US4610781A (en) * | 1983-12-30 | 1986-09-09 | Baxter Travenol Laboratories, Inc. | Fluid processing system with flow control manifold |
| WO1985004636A1 (en) * | 1984-04-12 | 1985-10-24 | Baxter Travenol Laboratories, Inc. | Disposable container having semi-rigid access end plug |
| EP0161551A2 (en) * | 1984-05-14 | 1985-11-21 | NPBI Nederlands Produktielaboratorium voor Bloedtransfusieapparatuur en Infusievloeistoffen B.V. | Apparatus for separating the blood plasma and the buffycoat from the blood in a flexible vessel |
| EP0161551A3 (en) * | 1984-05-14 | 1986-12-10 | Npbi Bv | Apparatus for separating the blood plasma and the buffycoat from the blood in a flexible vessel |
| US4705668A (en) * | 1985-03-27 | 1987-11-10 | Medica Corporation | Electrolyte analyzer |
| US4649028A (en) * | 1985-03-27 | 1987-03-10 | Medica Corporation | Electrolyte analyzer |
| US4675010A (en) * | 1985-10-04 | 1987-06-23 | American Omni Medical, Inc. | Thoracic drainage collection system and method |
| US4804363A (en) * | 1986-07-16 | 1989-02-14 | Autologous Blood Corporation | Apparatus and method for storing and processing blood |
| USRE33924E (en) * | 1986-07-16 | 1992-05-12 | Autologous Blood Corp. | Apparatus and method for storing and processing blood |
| US4917804A (en) * | 1986-10-31 | 1990-04-17 | Baxter International Inc. | Method and vessel for separation of cryoglobin |
| US4900321A (en) * | 1986-12-12 | 1990-02-13 | Baxter International Inc. | Set with integrally formed sample cell |
| US4820297A (en) * | 1986-12-12 | 1989-04-11 | Baxter International Inc. | Fluid delivery system with integrally formed sample cell |
| EP0272864A2 (en) * | 1986-12-23 | 1988-06-29 | Merck & Co. Inc. | Container device for separately storing and mixing two ingredients |
| US4731053A (en) * | 1986-12-23 | 1988-03-15 | Merck & Co., Inc. | Container device for separately storing and mixing two ingredients |
| EP0272864A3 (en) * | 1986-12-23 | 1988-09-07 | Merck & Co. Inc. | Container device for separately storing and mixing two ingredients |
| US4997083A (en) * | 1987-05-29 | 1991-03-05 | Vifor S.A. | Container intended for the separate storage of active compositions and for their subsequent mixing |
| US4915847A (en) * | 1987-08-04 | 1990-04-10 | Baxter International Inc. | Cryoglobulin separation |
| DE8716004U1 (en) * | 1987-12-03 | 1988-02-11 | Korth, Ruth, Dr.Med., 8000 Muenchen, De | |
| US5407425A (en) * | 1989-12-29 | 1995-04-18 | Werner; Margrit | System for the collecting and retransfusion of autologous blood |
| EP0438703A1 (en) * | 1989-12-29 | 1991-07-31 | Margrit Dipl.-Ing. Werner | System for the collection and retransfusion of autologous blood |
| US5030215A (en) * | 1990-01-03 | 1991-07-09 | Cryolife, Inc. | Preparation of fibrinogen/factor XIII precipitate |
| US5207509A (en) * | 1991-03-07 | 1993-05-04 | Fresenius Ag | Multichamber bag |
| WO1993021892A1 (en) * | 1992-04-23 | 1993-11-11 | Seroteknik Hb | System and method for centrifugal separation of two or more fractions from a composite liquid, and a bag intended therefor |
| EP0608882A1 (en) * | 1993-01-29 | 1994-08-03 | Terumo Kabushiki Kaisha | Apparatus for separation of liquid |
| US5456824A (en) * | 1993-01-29 | 1995-10-10 | Terumo Kabushiki Kaisha | Apparatus for separation of liquid |
| US20070179424A1 (en) * | 1994-12-05 | 2007-08-02 | Pablo Rubinstein | High concentration white cells, a method for agglomeration of the high concentration and a bag set for use in conjunction therewith |
| US7169547B2 (en) | 1994-12-05 | 2007-01-30 | New York Blood Center, Inc. | High concentration white blood cells as a therapeutic product |
| US6491678B1 (en) * | 1994-12-05 | 2002-12-10 | New York Blood Center, Inc. | Freezer bag |
| US5656154A (en) * | 1995-06-07 | 1997-08-12 | Organ, Inc. | Method and apparatus for separating a fluid into components and for washing a material |
| US5770069A (en) * | 1995-06-07 | 1998-06-23 | Organ, Inc. | Collapsible container for holding a fluid during a centrifugation operation |
| US6039720A (en) * | 1995-08-08 | 2000-03-21 | Gambro Ab | Bag for containing a sterile medical solution |
| US6039719A (en) * | 1995-08-08 | 2000-03-21 | Gambro Ab | Bag for containing a sterile medical solution and method of mixing a sterile medical solution |
| US5910138A (en) * | 1996-05-13 | 1999-06-08 | B. Braun Medical, Inc. | Flexible medical container with selectively enlargeable compartments and method for making same |
| US6764567B2 (en) | 1996-05-13 | 2004-07-20 | B. Braun Medical | Flexible medical container with selectively enlargeable compartments and method for making same |
| US5944709A (en) * | 1996-05-13 | 1999-08-31 | B. Braun Medical, Inc. | Flexible, multiple-compartment drug container and method of making and using same |
| WO1997042897A1 (en) * | 1996-05-13 | 1997-11-20 | B. Braun Medical | Flexible, multiple-compartment drug container and method of making and using same |
| JP2009062094A (en) * | 1996-05-13 | 2009-03-26 | B Braun Medical Inc | Flexible, multi-compartment drug container and method of making and using same |
| US6165161A (en) * | 1996-05-13 | 2000-12-26 | B. Braun Medical, Inc. | Sacrificial port for filling flexible, multiple-compartment drug container |
| US6996951B2 (en) | 1996-05-13 | 2006-02-14 | B. Braun Medical Inc. | Flexible multi-compartment container with peelable seals and method for making same |
| US6198106B1 (en) | 1996-05-13 | 2001-03-06 | B. Braun Medical, Inc. | Transport and sterilization carrier for flexible, multiple compartment drug container |
| US6203535B1 (en) | 1996-05-13 | 2001-03-20 | B. Braun Medical, Inc. | Method of making and using a flexible, multiple-compartment drug container |
| US6846305B2 (en) | 1996-05-13 | 2005-01-25 | B. Braun Medical Inc. | Flexible multi-compartment container with peelable seals and method for making same |
| US6468377B1 (en) | 1996-05-13 | 2002-10-22 | B. Braun Medical Inc. | Flexible medical container with selectively enlargeable compartments and method for making same |
| US5928213A (en) * | 1996-05-13 | 1999-07-27 | B. Braun Medical, Inc. | Flexible multiple compartment medical container with preferentially rupturable seals |
| US20030047467A1 (en) * | 1996-05-13 | 2003-03-13 | Smith Steven L. | Flexible multi-compartment container with peelable seals and method for making same |
| WO1997049959A3 (en) * | 1996-06-25 | 1998-03-19 | Thermogenesis Corp | Freezing and thawing bag, mold, apparatus and method |
| US6808675B1 (en) | 1996-06-25 | 2004-10-26 | Thermogenesis Corp. | Freezing and thawing bag, mold, apparatus and method |
| US6232115B1 (en) | 1996-06-25 | 2001-05-15 | Thermogenesis Corp. | Freezing and thawing bag, mold, apparatus and method |
| US6146124A (en) * | 1996-06-25 | 2000-11-14 | Thermogenesis Corp. | Freezing and thawing bag, mold, apparatus and method |
| EP1037565A4 (en) * | 1997-11-12 | 2004-05-06 | Braun Medical Inc | Flexible medical container with selectively enlargeable compartments and method for making same |
| EP1037565A1 (en) * | 1997-11-12 | 2000-09-27 | B. Braun Medical, Inc. | Flexible medical container with selectively enlargeable compartments and method for making same |
| US6183460B1 (en) * | 1998-01-22 | 2001-02-06 | Baxter International Inc. | Multi-use solution container having flaps |
| US6113575A (en) * | 1998-05-14 | 2000-09-05 | Terumo Cardiovascular Systems Corporation | Volume control apparatus for a flexible venous reservoir |
| US7036986B2 (en) * | 1998-05-29 | 2006-05-02 | Toyo Seikan Kaisha, Ltd. | Pouch having a branched chamber |
| US20040045842A1 (en) * | 1998-05-29 | 2004-03-11 | Naoto Matsuda | Pouch having a branched chamber |
| US9005551B2 (en) * | 1998-06-24 | 2015-04-14 | Roche Molecular Systems, Inc. | Sample vessels |
| US20110143968A1 (en) * | 1998-06-24 | 2011-06-16 | Iquum, Inc. | Sample vessels |
| US10022722B2 (en) | 1998-06-24 | 2018-07-17 | Roche Molecular Systems, Inc. | Sample vessels |
| US7992725B2 (en) | 2002-05-03 | 2011-08-09 | Biomet Biologics, Llc | Buoy suspension fractionation system |
| US7837884B2 (en) | 2002-05-03 | 2010-11-23 | Hanuman, Llc | Methods and apparatus for isolating platelets from blood |
| US7470371B2 (en) | 2002-05-03 | 2008-12-30 | Hanuman Llc | Methods and apparatus for isolating platelets from blood |
| US8950586B2 (en) | 2002-05-03 | 2015-02-10 | Hanuman Llc | Methods and apparatus for isolating platelets from blood |
| US8187477B2 (en) | 2002-05-03 | 2012-05-29 | Hanuman, Llc | Methods and apparatus for isolating platelets from blood |
| US7832566B2 (en) | 2002-05-24 | 2010-11-16 | Biomet Biologics, Llc | Method and apparatus for separating and concentrating a component from a multi-component material including macroparticles |
| US7914689B2 (en) | 2002-05-24 | 2011-03-29 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US9897589B2 (en) | 2002-05-24 | 2018-02-20 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US7780860B2 (en) | 2002-05-24 | 2010-08-24 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US10183042B2 (en) | 2002-05-24 | 2019-01-22 | Biomet Manufacturing, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US7845499B2 (en) | 2002-05-24 | 2010-12-07 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US7374678B2 (en) | 2002-05-24 | 2008-05-20 | Biomet Biologics, Inc. | Apparatus and method for separating and concentrating fluids containing multiple components |
| US9114334B2 (en) | 2002-05-24 | 2015-08-25 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US8062534B2 (en) | 2002-05-24 | 2011-11-22 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US10393728B2 (en) | 2002-05-24 | 2019-08-27 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US8808551B2 (en) | 2002-05-24 | 2014-08-19 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US8603346B2 (en) | 2002-05-24 | 2013-12-10 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US8048321B2 (en) | 2002-05-24 | 2011-11-01 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US8163184B2 (en) | 2002-05-24 | 2012-04-24 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US10328404B2 (en) | 2005-04-22 | 2019-06-25 | Life Technologies Corporation | Gas spargers and related container systems |
| US7771590B2 (en) | 2005-08-23 | 2010-08-10 | Biomet Manufacturing Corp. | Method and apparatus for collecting biological materials |
| US20070075016A1 (en) * | 2005-08-23 | 2007-04-05 | Biomet Manufacturing Corp. | Method and apparatus for collecting biological materials |
| US8512575B2 (en) | 2005-08-23 | 2013-08-20 | Biomet Biologics, Llc | Method and apparatus for collecting biological materials |
| US8048297B2 (en) | 2005-08-23 | 2011-11-01 | Biomet Biologics, Llc | Method and apparatus for collecting biological materials |
| US8236258B2 (en) | 2005-08-23 | 2012-08-07 | Biomet Biologics, Llc | Method and apparatus for collecting biological materials |
| US20070208321A1 (en) * | 2005-08-23 | 2007-09-06 | Biomet Manufacturing Corp. | Method And Apparatus For Collecting Biological Materials |
| US20100255977A1 (en) * | 2005-08-23 | 2010-10-07 | Biomet Manufacturing Corp. | Method and Apparatus for Collecting Biological Materials |
| US8048320B2 (en) | 2005-08-23 | 2011-11-01 | Biomet Manufacturing Corp. | Method and apparatus for collecting biological materials |
| US8567609B2 (en) | 2006-05-25 | 2013-10-29 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US20100047403A1 (en) * | 2006-10-30 | 2010-02-25 | Elizabeth Johnson | Pouch container for food product |
| US8119013B2 (en) | 2007-04-12 | 2012-02-21 | Hanuman, Llc | Method of separating a selected component from a multiple component material |
| US8596470B2 (en) | 2007-04-12 | 2013-12-03 | Hanuman, Llc | Buoy fractionation system |
| US7806276B2 (en) | 2007-04-12 | 2010-10-05 | Hanuman, Llc | Buoy suspension fractionation system |
| US9138664B2 (en) | 2007-04-12 | 2015-09-22 | Biomet Biologics, Llc | Buoy fractionation system |
| US9649579B2 (en) | 2007-04-12 | 2017-05-16 | Hanuman Llc | Buoy suspension fractionation system |
| US8328024B2 (en) | 2007-04-12 | 2012-12-11 | Hanuman, Llc | Buoy suspension fractionation system |
| US20100198185A1 (en) * | 2007-06-21 | 2010-08-05 | Goerisch Irmgard | Multi-chamber bag for use with enteral nutrition |
| US20110022022A1 (en) * | 2007-07-19 | 2011-01-27 | Tatsuro Tsuruoka | Multi-chamber bag |
| US8845611B2 (en) * | 2007-07-19 | 2014-09-30 | Otsuka Pharmaceutical Factory, Inc. | Multi-chamber bag |
| US10213294B2 (en) | 2007-12-07 | 2019-02-26 | Keller Medical, Inc. | Apparatus for use in a surgical procedure |
| US10463472B2 (en) | 2007-12-07 | 2019-11-05 | Keller Medical, Inc. | Apparatus for use in a surgical procedure |
| US11253351B2 (en) | 2007-12-07 | 2022-02-22 | Keller Medical, Inc. | Apparatus for use in a surgical procedure |
| US10400017B2 (en) | 2008-02-27 | 2019-09-03 | Biomet Biologics, Llc | Methods and compositions for delivering interleukin-1 receptor antagonist |
| US11725031B2 (en) | 2008-02-27 | 2023-08-15 | Biomet Manufacturing, Llc | Methods and compositions for delivering interleukin-1 receptor antagonist |
| US9701728B2 (en) | 2008-02-27 | 2017-07-11 | Biomet Biologics, Llc | Methods and compositions for delivering interleukin-1 receptor antagonist |
| US9719063B2 (en) | 2008-02-29 | 2017-08-01 | Biomet Biologics, Llc | System and process for separating a material |
| US8337711B2 (en) | 2008-02-29 | 2012-12-25 | Biomet Biologics, Llc | System and process for separating a material |
| US8801586B2 (en) * | 2008-02-29 | 2014-08-12 | Biomet Biologics, Llc | System and process for separating a material |
| US20130196425A1 (en) * | 2008-02-29 | 2013-08-01 | Biomet Biologics, Llc | System and Process for Separating a Material |
| US20110274849A1 (en) * | 2009-01-23 | 2011-11-10 | Maarten Van Pul | Packaging for two or more fluids |
| US8783470B2 (en) | 2009-03-06 | 2014-07-22 | Biomet Biologics, Llc | Method and apparatus for producing autologous thrombin |
| US8992862B2 (en) | 2009-04-03 | 2015-03-31 | Biomet Biologics, Llc | All-in-one means of separating blood components |
| US8313954B2 (en) | 2009-04-03 | 2012-11-20 | Biomet Biologics, Llc | All-in-one means of separating blood components |
| WO2010122542A1 (en) * | 2009-04-20 | 2010-10-28 | Fuil Technologies Limited | A container for storing a flowable bodily material, and a method for storing a flowable bodily material |
| US20160038275A1 (en) * | 2009-04-29 | 2016-02-11 | Keller Medical, Inc. | Silicone breast implant delivery |
| US10058415B2 (en) * | 2009-04-29 | 2018-08-28 | Keller Medical, Inc. | Silicone breast implant delivery |
| US9011800B2 (en) | 2009-07-16 | 2015-04-21 | Biomet Biologics, Llc | Method and apparatus for separating biological materials |
| US9533090B2 (en) | 2010-04-12 | 2017-01-03 | Biomet Biologics, Llc | Method and apparatus for separating a material |
| US8591391B2 (en) | 2010-04-12 | 2013-11-26 | Biomet Biologics, Llc | Method and apparatus for separating a material |
| US9239276B2 (en) | 2011-04-19 | 2016-01-19 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US10934514B2 (en) | 2011-09-29 | 2021-03-02 | Life Technologies Corporation | Filter systems for separating microcarriers from cell culture solutions |
| US11840684B2 (en) | 2011-09-29 | 2023-12-12 | Life Technologies Corporation | Filter systems for separating microcarriers from cell culture solutions |
| US10843141B2 (en) | 2011-09-30 | 2020-11-24 | Life Technologies Corporation | Container with film sparger |
| US10350554B2 (en) | 2011-09-30 | 2019-07-16 | Life Technologies Corporation | Container with film Sparger |
| US9642956B2 (en) | 2012-08-27 | 2017-05-09 | Biomet Biologics, Llc | Apparatus and method for separating and concentrating fluids containing multiple components |
| US10441634B2 (en) | 2013-03-15 | 2019-10-15 | Biomet Biologics, Llc | Treatment of peripheral vascular disease using protein solutions |
| US9950035B2 (en) | 2013-03-15 | 2018-04-24 | Biomet Biologics, Llc | Methods and non-immunogenic compositions for treating inflammatory disorders |
| US10143725B2 (en) | 2013-03-15 | 2018-12-04 | Biomet Biologics, Llc | Treatment of pain using protein solutions |
| US9895418B2 (en) | 2013-03-15 | 2018-02-20 | Biomet Biologics, Llc | Treatment of peripheral vascular disease using protein solutions |
| US10576130B2 (en) | 2013-03-15 | 2020-03-03 | Biomet Manufacturing, Llc | Treatment of collagen defects using protein solutions |
| US10208095B2 (en) | 2013-03-15 | 2019-02-19 | Biomet Manufacturing, Llc | Methods for making cytokine compositions from tissues using non-centrifugal methods |
| US9556243B2 (en) | 2013-03-15 | 2017-01-31 | Biomet Biologies, LLC | Methods for making cytokine compositions from tissues using non-centrifugal methods |
| US20160000652A1 (en) * | 2014-02-07 | 2016-01-07 | Eurozyto Gmbh | Container and kit for providing parenteral nutrition |
| US9968519B2 (en) * | 2014-06-26 | 2018-05-15 | Advanced Scientifics, Inc. | Freezer bag, storage system, and method of freezing |
| US20150374583A1 (en) * | 2014-06-26 | 2015-12-31 | Advanced Scientifics, Inc. | Freezer bag, storage system, and method of freezing |
| US9993748B2 (en) * | 2014-08-11 | 2018-06-12 | Auxocell Laboratories, Inc. | Centrifuge clip and method |
| US10441901B2 (en) | 2014-08-11 | 2019-10-15 | Auxocell Laboratories, Inc. | Centrifuge clip and method |
| US20160038946A1 (en) * | 2014-08-11 | 2016-02-11 | Auxocell Laboratories, Inc. | Centrifuge clip and method |
| US10589197B2 (en) | 2016-12-01 | 2020-03-17 | Life Technologies Corporation | Microcarrier filter bag assemblies and methods of use |
| US11344827B2 (en) | 2016-12-01 | 2022-05-31 | Life Technologies Corporation | Microcarrier filter bag assemblies and methods of use |
| US11890557B2 (en) | 2016-12-01 | 2024-02-06 | Life Technologies Corporation | Microcarrier filter bag assemblies and methods of use |
| US20210187495A1 (en) * | 2017-06-22 | 2021-06-24 | Texas Heart Institute | Multi-compartment biological fluid storage device |
| CN111617887A (en) * | 2019-02-28 | 2020-09-04 | 赛默电子Led有限公司 | Extruder and extruder system for separating components of biological suspensions and methods of use |
| US20200306421A1 (en) * | 2019-04-01 | 2020-10-01 | Sterigear, Llc | Dual drainage bag, assemblies, and related methods |
| US11730875B2 (en) * | 2019-04-01 | 2023-08-22 | Sterigear, Llc | Dual drainage bag, assemblies, and related methods |
| US11957733B2 (en) | 2019-10-28 | 2024-04-16 | Biomet Manufacturing, Llc | Treatment of collagen defects using protein solutions |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US3545671A (en) | Apparatus for and method of collecting,storing,separating and dispensing blood and blood components | |
| US3187750A (en) | Multiple bag blood storage unit | |
| USRE27359E (en) | Method and apparatus for treating blood | |
| US4994021A (en) | Apparatus and method for collecting and freezing blood plasma | |
| US2702034A (en) | Apparatus for collecting, storing, and dispensing whole blood | |
| US4608043A (en) | I.V. fluid storage and mixing system | |
| US3064647A (en) | Blood component separation method and apparatus | |
| US4040959A (en) | Multi-purpose blood bag | |
| US5562836A (en) | Method for storing blood in a container having multiple chambers | |
| US4198972A (en) | Blood and blood component storage bags | |
| US3911918A (en) | Blood collection, storage and administering bag | |
| CA1126606A (en) | Method and apparatus for collecting blood plasma | |
| CA1316821C (en) | Sterilizable system for blood storage | |
| US5462526A (en) | Flexible, sterile container and method of making and using same | |
| US6935492B1 (en) | Flexible mixing pouch with aseptic burstable internal chambers | |
| US5403304A (en) | Blood collection device | |
| US4365629A (en) | Platelet freezing bag | |
| US3006341A (en) | Medical fluids handling and administering apparatus | |
| EP0276102A2 (en) | Baby feeding packs | |
| EP0596497B1 (en) | Bag for containing at least two separate substances that are to be mixed | |
| KR100191138B1 (en) | A container for receiving and separating a fluid, preferably blood plasma, into its ingredients | |
| US3566930A (en) | Means for sterilely transferring blood plasma, serum, biological or pharmaceutical fluids, and the like | |
| EP0176590A1 (en) | Container such as a nursing container having a protection compartment for a dispensing member | |
| US5257986A (en) | Container for the separate sterile storage of at least two substances and for mixing said substances | |
| JPS61500219A (en) | Multi-chamber container with leak detection compartment |