WO1997006837A1 - Perforated artificial skin grafts - Google Patents
Perforated artificial skin grafts Download PDFInfo
- Publication number
- WO1997006837A1 WO1997006837A1 PCT/US1996/013244 US9613244W WO9706837A1 WO 1997006837 A1 WO1997006837 A1 WO 1997006837A1 US 9613244 W US9613244 W US 9613244W WO 9706837 A1 WO9706837 A1 WO 9706837A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- wound
- membrane
- layer
- multilayer membrane
- multilayer
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/60—Materials for use in artificial skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/10—Hair or skin implants
- A61F2/105—Skin implants, e.g. artificial skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/22—Polypeptides or derivatives thereof, e.g. degradation products
- A61L27/24—Collagen
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2310/00—Prostheses classified in A61F2/28 or A61F2/30 - A61F2/44 being constructed from or coated with a particular material
- A61F2310/00005—The prosthesis being constructed from a particular material
- A61F2310/00365—Proteins; Polypeptides; Degradation products thereof
Definitions
- bilayer membrane (Yannas et al . , U.S. Patent 4,060,081).
- the bilayer membrane comprises a first layer formed from a crosslinked collagen-glycosaminoglycan composite and a moisture transmission control layer formed from a nontoxic material.
- the moisture transmission control layer provides the multilayer membrane with a controlled moisture flux.
- the multilayer membrane not only provides immediate wound closure, but also builds neodermis, thus permitting the satisfactory use of a thin epidermal autograft (or cultured epidermal cells) rather than a thick conventional auto ⁇ graft. It also results in less hypertrophic scar forma- tion, thereby yielding cosmetic outcomes comparable to or better than conventional autograft techniques.
- bilayer membranes and other temporary wound coverings are compromised by the high rate of infections associated with their use.
- the control of infection in burn wounds covered with the bilayer membrane or other temporary coverings would significantly advance the ability to successfully treat patients with severe and extensive burns.
- the present invention is based on the unexpected discovery that perforations (also referred to herein as "meshings") in multilayer membranes, used as synthetic skin to repair burn wounds, can significantly reduce the inci ⁇ dence of infection at the wound site and also increase the extent at which the graft will adhere to or "take” to the wound.
- the multilayer membrane comprises a porous biodegradable polymeric mem ⁇ brane having a moisture control layer disposed thereon.
- the porous biodegradable polymeric membrane typically has (1) controllable biodegradability in the presence of body enzymes; (2) has controllable solubility in the presence of bodily fluids; (3) is substantially noni munogenic upon grafting or implantation; (4) provokes no substantial foreign body response upon grafting or implantation; and (5) promotes the adherence and proliferation of cells, such as fibroblasts and endothelial cells.
- perforations in the multilayer membrane allow pus and exudate to drain from the wound site while still pro ⁇ viding the moisture transmission control layer with suffi ⁇ cient moisture impermeability to prevent significant mois- ture loss from the wound.
- Use of perforated multilayer membranes to treat burn wounds leads to significantly lower incidence of infection compared with unperforated mem ⁇ branes. When infections occur in wounds covered by a perforated multilayer membrane, they are generally of reduced severity. Perforated multilayer membranes also "take”, i.e. adhere to and become permanently fixed to the wound bed, more completely than multilayer membranes which lack the perforations.
- Figure 1 illustrates a non-expandable multilayer membrane with a multiplicity of aligned, non-overlapping slit perforations.
- Figure 2 illustrates an expandable multilayer membrane with a multiplicity of staggered, overlapping slit perfora- tions.
- Figure 3 illustrates a multilayer membrane with a multiplicity of cross-slitted perforations arranged in a rectangular pattern.
- Figure 5 illustrates schematically a multilayer mem ⁇ brane as described herein, wherein the perforations are not shown.
- Moisture transmission control layers such as those described in U.S. Patent No. 4,060,081, have been used in artificial skin to control the rate of body moisture loss and heat loss from the damaged skin area. Although this layer is important in homeostasis and protecting the wound area from mechanical abrasion, it can also trap exudate from the wound. Infection or exudate can decrease the ability of an artificial skin graft to "take" to the wound site, i.e. to adhere to the wound. Infections develop and spread because wound exudate and pus in infected areas is trapped under the moisture transmission control layer and thus spread laterally.
- the present invention provides a means for significantly reducing and/or eliminating this type of infection. It has been discovered that if the multilayer membrane is perforated, exudate and pus can drain away from the wound site and relieve hydrostatic pressure. Typically, the exudate is absorbed by absorbent dressings which are used to cover the artificial skin.
- the perforations are constructed in a manner to sub ⁇ stantially prevent the passage of fluids and water vapor in the absence of hydrostatic pressure.
- the perforations in the multilayer membrane have a size and shape and are arranged in a pattern such that the membrane is permeable to fluid in the presence of hydrostatic pressure from exudate in the wound.
- the size, shape and pattern of the perforations are chosen so that the moisture loss from a wound to which the multilayer membrane has been applied is maintained below about 2.2 mg/cm 2 /hour, and preferably between about 0.1 mg/cm 2 /hour and about 1.0 mg/cm 2 /hour.
- the perforations comprise a multiplicity or plurality of slits.
- the slits can be arranged in a wide variety of patterns. It is preferred that the slit pattern results in a non-expandable multi ⁇ layer membrane. Alternatively, the slit pattern can result in a multilayer membrane which expands upon the application of a lateral force to the multilayer membrane.
- An ex- pandable multilayer membrane can be stretched along at least one lateral axis.
- a "lateral axis" is an axis or line which traverses the entire cross section of a multi ⁇ layer membrane. When an expandable membrane is pulled in opposite directions at each end of the lateral axis, the membrane is stretched, thereby resulting in an increased surface area.
- An increase in surface area has the unde ⁇ sired effect of pulling the slits open to form permanent openings which will expose the underlying wound to in ⁇ creased moisture loss and external pathogens.
- it is essential to maintain the integrity of the membrane to optimize the proper function of the moisture control barri ⁇ er. Consequently, an expandable membrane is applied to a wound and maintained under conditions which prevent expan ⁇ sion or increases in the surface area of the membrane.
- a membrane which does not stretch or increase its surface area when pulled in opposite directions along a lateral axis is said to resist expansion along that lateral axis.
- a non-expandable multilayer membrane resists expan ⁇ sion along any lateral axis.
- a multilayer membrane having a multiplicity of slits resists expansion along a lateral axis when the lateral axis is not intersected by any slit.
- this type of multilayer membrane has a continuous band of membrane along the lateral axis that is free of slits.
- a non-expandable multilayer membrane has at least two perpen- dicular continuous bands of membrane in which the slits do not intersect. As a result, the membrane can be pulled in opposite directions along any lateral axis without stretch ⁇ ing or increasing the surface area of the multilayer mem- brane.
- FIG. 1 One example of a multilayer membrane which resists expansion is shown in Figure 1.
- the slits are arranged in non-overlapping parallel rows with the slits in each row being parallel to one another.
- a continuous band of mem- brane in which the slits do not intersect runs the length of the membrane.
- the slits are of equal length, are aligned with the slits in the same row, and are aligned with a slit in the adjacent row.
- Dimensions a, b and c in Figure 1 typically range from about 0.5 mm to about 5.0 mm in length. In one example, a is 1.7 mm, b is 1.1 mm and c is 2.7 mm.
- a multilayer membrane is expandable along a first lateral axis when at least one slit intersects each lateral axis parallel to the first lateral axis.
- the slits are arranged in non-overlapping parallel rows with the slits in each row being parallel to one another.
- the slits are of equal length and staggered with respect to the other slits in the row such that there is no continuous band of mem- brane between the rows which runs the length of the multi ⁇ layer membrane sample.
- the membrane will expand when opposing forces are applied at opposite ends of a lateral axis running perpendicular to the slits.
- This type of expandable membrane is shown in Figure 2.
- the degree of expansion is controlled by the length of the slits and the number of overlapping slits.
- Expandable membranes can be stretched, for example, to about 1.5, 2.0, 2.5 and 3.0 times their normal ⁇ urface area.
- the perforations comprise a plurality of cross-slits.
- Cross-slits can open like a valve, thereby maximizing fluid flow away from the wound site.
- the cross-slits can be arranged so that the membrane is expandable or is non-expandable.
- the cross-slits can be arranged into regular or irregular patterns, however regular patterns in which the slits are evenly spaced are preferred. Suitable patterns include polygonal patterns such trigonal, rectangular (see Figure 3) and hexagonal patterns.
- the first layer 10 comes into direct contact with the subcutaneous tissue or wound bed, there are three essential characteristics required of this layer. These are: insolubility in body fluids; ability to promote the adherence and proliferation of cell ⁇ , such as fibro- blasts and endothelial cells; a controlled rate of biodeg- radation such that the material provides a scaffold suit ⁇ able for wound repair; and nonimmunogenicity.
- These multi ⁇ layer membranes also include at least one additional layer, which has the primary function of controlling the moisture flux for the overall membrane.
- moisture transmission control layer 12 is illustrated in Figure 5 as being di ⁇ rectly bonded to the first layer. It should be understood, however, that additional layers can be added on top of layer 12 or between first layer 10 and moisture control layer 12 as long as such additional layers do not interfere with the essential functions of layers 10 and 12.
- the first layer is preferably a porous biodegradable polymeric membrane layer comprising a composite formed from collagen molecules that are crosslinked and covalently bonded with glycosa inoglycan (GAG) .
- GAG glycosa inoglycan
- specific glycosaminoglycans include but are not limited to chondroi- tin 6-sulfate, chondroitin 4-sulfate, heparan, heparan sulfate, keratan sulfate and dermatan sulfate.
- anionic polymers such as chitin and chitosan are suitable.
- the average pore size of the biodegradable first layer is within the range of about 9 ⁇ m to about 630 ⁇ m, preferably about 20 ⁇ m to 200 ⁇ m.
- the average pore size can be calcu- lated by stereology from scanning electron micrograph of the surface or cross section as described by Dagalakis et al . J . of Biomedical Materials Research 14:511 (1980). Materials which do not come within these parameters do not delay or arrest skin wound contraction and thus tend to induce synthesis of undesirable scar tissue, while those materials having pore sizes within the desired upper and lower limits have been found to effectively delay or arrest skin wound contraction and induce synthesis of new func ⁇ tional tissue.
- pore volume frac ⁇ tion of the first layer Another determining factor in the effec- tiveness of multilayer membrane ⁇ is the pore volume frac ⁇ tion of the first layer. This value is defined as the percentage of the total volume of the material which is occupied by pore space. A more detailed definition is given in Fischmeister, H.F. Proceedings Int. Symp. RILEM/I- UPAC, Moscow, September 18-21, 1973, Final Report Part II, p. C-439, the entire teachings of which are incorporated herein by reference. A high pore volume fraction in the first layer has been found to be clinically desirable, with pore volume fractions above about 80% being preferred. The degree of crosslink density is an important param ⁇ eter of this invention since it is a direct, controlling factor in the biodegradation rate of the material.
- the degree of crosslinking has been determined to be about 140 enzyme units (e.u.), and is preferably below about 120 e.u.
- the crosslinked composites should have an average molecular weight between crosslinks, (M c ) , of between about 800 and about 60,000 daltons. Composites with an M c of between 10,000 and about 40,000 tend to have the best balance between physical and thera ⁇ Therapeutic properties and are this preferred.
- a preferred method for covalently crosslinking the collagen-GAG composites is known as aldehyde crosslinking.
- the resulting crosslinked collagen-glycosaminoglycan composite has a rate of biodegradation which is low enough to enable the compos ⁇ ite to be a suitable scaffold for wound repair.
- the maxi ⁇ mum degradation rate has been determined to be about 140 enzyme units (e.u.), measured as described in Yannas et al . , U.S. Patent No. 4,947,840.
- the biodegra- dation rate is below about 120 e.u.
- Unbanded structures are characterized by the absence of periodic banding at 640A, characteristic of native collagen, when viewed by transmission electron microscopy (Sylvester et al . , Thrombosis Research 55:135 (1989)).
- Unbanded struc ⁇ tures can be obtained by crosslinking at pHs below about 4.25, preferably at about 3.0.
- Covalent crosslinking can be achieved by other specif ⁇ ic techniques including radiation and dehydrother al meth ⁇ ods.
- An example of a suitable crosslinking technique is to treat collagen with 0.25% aqueous glutaraldehyde solu- tion in 0.05 M acetic acid for twenty four hours at 20- 25°C.
- These techniques are discussed in greater detail in Yannas et ai., U.S. Patent No. 4,060,081, Yannas and Kirk, U.S. Patent No. 4,448,718 and Yannas et al . , U.S. Patent No. 4,947,840, the entire teachings of which have been incorporated herein by reference.
- Other suitable chemical crosslinking techniques include carbodiimide coupling, azide coupling and diisocyanate crosslinking.
- Particularly preferred first layer materials are crosslinked collagen-glycosaminoglycan composites contain- ing between about 6% and about 15% of a sulfate-containing mucopolysaccharide and crosslinked to an M c value of be ⁇ tween about 5,000 and about 10,000. Chondroitin 6-sulfate forms especially outstanding composites.
- the other essential property of this layer is that it be nontoxic.
- the material should contain no toxic sub- stances capable of diffusing out into tissues contacting a multilayer membrane graft or capable of being extracted therefrom. Also, the material should be capable of resist ⁇ ing enzymatic degradation or other degradation resulting from contact with other layers of the membrane or with tissue which degradation might lead to the production of substances that are toxic to neighboring tissue.
- the moisture-control layer adhere to the wet surface of the first layer with a bond shear strength of at least about 10 psi, and preferably about 100 psi. It also is desirable that it have mechanical properties of: Young's modulus in the range of from about 100 to 1,000 psi; ultimate tensile strength of from about 100 to about 1,000 psi; and elonga ⁇ tion at break of from about 20 to about 100%
- the moisture control layer is capable of being sterilized, i.e., of being subjected to physical or chemical treatment that kills bacteria and bacterial spores on its surface.
- Suit ⁇ able sterilization techniques include dry heat, exposure to ethylene oxide, irradiation, immersion in glutaraldehyde solution, etc.
- Silicone polymer is preferred as the moisture control layer. It is available as a non-toxic product in a care- fully controlled medical grade. Its flow properties are of thixotropic nature, permitting uniform application by knife blade onto the surface of collagen-glycosaminoglycan com ⁇ posite layer with controlled penetration into the latter. Curing can be done at 100% relative humidity, thereby avoiding dehydration of the lower layer, consisting of the collagen-glycosaminoglycan composite, and preventing defor ⁇ mation of the multilayered structure. Silastic Medical Grade silicone typically has 180° peel strength, between 6 and 16 g/cm.
- a layer of gauze or other fabric or mesh could be usefully employed.
- Cotton or other textile mesh can be incorporated as a reinforcing mechanism by placing the textile material over the collagen-glycosaminoglycan composite and applying the Silastic silicone over the mesh onto the collagen-glycos- a inoglycan surface by knife coating. Curing at room temperature and 100% relative humidity overnight (16-24 hours) can result in a reinforced composite which is some ⁇ what stiffer than one without the mesh but with substan ⁇ tially improved tensile strength.
- the optimum thickness of a synthetic skin is related to the following parameters: (1) thickness of the skin to be replaced; (2) nature of wound and dimensions; (3) thickness of top layer required to control moisture flux; and, (4) relation of suturability and drapability to thick- ness.
- the artificial skin was applied to the wound so that the collagen template layer was in direct contact with the excised wound.
- the silicone layer (identified by the black threads) was placed out (away from the wound bed) .
- the material readily adhered and conformed to the wound sur ⁇ face. Any air bubbles were carefully removed by moving them to the edge of the sheet.
- the artificial skin ⁇ heets were secured by staples or sutures placed in an interrupted fashion (with fine synthetic monofilament suture, or 4/0 or 5/0 chromic, using a fine atraumatic needle) under slight tension. Care was taken not to spread or expand the mem ⁇ brane and to achieve a primary closure between the artifi- cial skin and adjacent unburned skin or between sheets of the artificial skin. Each strip of artificial skin was sutured or stapled in place independently.
- the area was covered with an inner dressing consisting of a single layer of wide mesh gauze, secured by staples or sutures to the normal tissue at the edges of the grafted area. This layer was then wrapped with an outer dressing consisting of two or three layers of 4 inch (10.2 cm) wide rolled gauze.
- Descriptive statistics are given for all entry, treat ⁇ ment, and outcome characteristics. Frequencies and confi ⁇ dence intervals were used to summarize the infection and culture results. Percentages were used to summarize physi ⁇ cian assessments at each follow-up visit. Confidence intervals for dichotomous data were computed using the binomial distribution. The method of Bickel and Doksum, Mathematical Statistics - Basic Ideas and Selected Topic, Holden-Day, San Francisco, 180-2 (1977) , was used for the calculations. Confidence intervals for continuous data were computed for both the mean and the median. Confidence intervals for the median were based on the Sign Test (Hollander and Wolfe, Nonparametric Statistical Meth- ods , Wiley, New York, 48-9 (1976)).
Abstract
Disclosed is a perforated multilayer membrane useful as artificial skin. The multilayer membrane comprises a porous biodegradable polymeric membrane having a moisture control layer disposed thereon. The moisture control layer is perforated such that the multilayer membrane is permeable to fluid in the presence of hydrostatic pressure from exudate in the wound while being substantially impermeable to fluid and water vapor in the wound in the absence of hydrostatic pressure from exudate in the wound. Also disclosed is a method of covering a burn or wound with the perforated multilayer membrane.
Description
PERFORATED ARTIFICIAL SKIN GRAFTS
Background
Each year there are approximately two million patients with burns requiring medical attention in the United States. Of these injuries, there are roughly 130,000 hospital admissions, of which about 20,000 are considered life-threatening. Successful treatment requires rapid covering of the burn wound. The wound cover of choice is conventional autograft; however, burn wound management is frequently hampered by the lack of availability of a suit¬ able quantity of donor skin from the patient.
Recent advancements in burn treatment have made use of artificial skin. One of the more successful is a bilayer membrane (Yannas et al . , U.S. Patent 4,060,081). The bilayer membrane comprises a first layer formed from a crosslinked collagen-glycosaminoglycan composite and a moisture transmission control layer formed from a nontoxic material. The moisture transmission control layer provides the multilayer membrane with a controlled moisture flux. The multilayer membrane not only provides immediate wound closure, but also builds neodermis, thus permitting the satisfactory use of a thin epidermal autograft (or cultured epidermal cells) rather than a thick conventional auto¬ graft. It also results in less hypertrophic scar forma- tion, thereby yielding cosmetic outcomes comparable to or better than conventional autograft techniques.
The use of bilayer membranes and other temporary wound coverings is compromised by the high rate of infections associated with their use. The control of infection in burn wounds covered with the bilayer membrane or other temporary coverings would significantly advance the ability to successfully treat patients with severe and extensive burns.
Sum ary of the Invention
The present invention is based on the unexpected discovery that perforations (also referred to herein as "meshings") in multilayer membranes, used as synthetic skin to repair burn wounds, can significantly reduce the inci¬ dence of infection at the wound site and also increase the extent at which the graft will adhere to or "take" to the wound.
One embodiment of the present invention is a multi- layer membrane useful as artificial skin. The multilayer membrane comprises a porous biodegradable polymeric mem¬ brane having a moisture control layer disposed thereon. The porous biodegradable polymeric membrane typically has (1) controllable biodegradability in the presence of body enzymes; (2) has controllable solubility in the presence of bodily fluids; (3) is substantially noni munogenic upon grafting or implantation; (4) provokes no substantial foreign body response upon grafting or implantation; and (5) promotes the adherence and proliferation of cells, such as fibroblasts and endothelial cells. The moisture control layer is perforated such that the multilayer membrane is permeable to fluid in the presence of hydrostatic pressure from exudate in the wound while being substantially imper¬ meable to fluid and water vapor in the wound in the absence of hydrostatic pressure from exudate in the wound.
Another embodiment of the present invention is a method of covering a full thickness or partial thickness burn or other wound site on a human or animal. The method comprises applying the multilayer membrane described above to the burn or wound site.
The perforations in the multilayer membrane allow pus and exudate to drain from the wound site while still pro¬ viding the moisture transmission control layer with suffi¬ cient moisture impermeability to prevent significant mois- ture loss from the wound. Use of perforated multilayer
membranes to treat burn wounds leads to significantly lower incidence of infection compared with unperforated mem¬ branes. When infections occur in wounds covered by a perforated multilayer membrane, they are generally of reduced severity. Perforated multilayer membranes also "take", i.e. adhere to and become permanently fixed to the wound bed, more completely than multilayer membranes which lack the perforations.
Brief Description of the Drawings Figure 1 illustrates a non-expandable multilayer membrane with a multiplicity of aligned, non-overlapping slit perforations.
Figure 2 illustrates an expandable multilayer membrane with a multiplicity of staggered, overlapping slit perfora- tions.
Figure 3 illustrates a multilayer membrane with a multiplicity of cross-slitted perforations arranged in a rectangular pattern.
Figure 4 illustrates a multilayer membrane with a multiplicity of hole perforations arranged in a trigonal pattern.
Figure 5 illustrates schematically a multilayer mem¬ brane as described herein, wherein the perforations are not shown.
Detailed Description of the Invention
Moisture transmission control layers, such as those described in U.S. Patent No. 4,060,081, have been used in artificial skin to control the rate of body moisture loss and heat loss from the damaged skin area. Although this layer is important in homeostasis and protecting the wound area from mechanical abrasion, it can also trap exudate from the wound. Infection or exudate can decrease the ability of an artificial skin graft to "take" to the wound
site, i.e. to adhere to the wound. Infections develop and spread because wound exudate and pus in infected areas is trapped under the moisture transmission control layer and thus spread laterally. The present invention provides a means for significantly reducing and/or eliminating this type of infection. It has been discovered that if the multilayer membrane is perforated, exudate and pus can drain away from the wound site and relieve hydrostatic pressure. Typically, the exudate is absorbed by absorbent dressings which are used to cover the artificial skin.
The perforations are constructed in a manner to sub¬ stantially prevent the passage of fluids and water vapor in the absence of hydrostatic pressure. The perforations in the multilayer membrane have a size and shape and are arranged in a pattern such that the membrane is permeable to fluid in the presence of hydrostatic pressure from exudate in the wound. The size, shape and pattern of the perforations are chosen so that the moisture loss from a wound to which the multilayer membrane has been applied is maintained below about 2.2 mg/cm2/hour, and preferably between about 0.1 mg/cm2/hour and about 1.0 mg/cm2/hour.
The perforations are preferably spaced as close to¬ gether as possible to minimize the lateral path for exudate to spread within the wound site. The total open area of the perforations is preferably small to minimize moisture loss in the absence of hydrostatic pressure, and also to maximize the resistance to fluid flow at low hydrostatic pressures. The perforations penetrate completely through the moisture transmission control layer. Optionally, the perforations penetrate either partially or completely through the porous biodegradable polymeric membrane layer. However, it is preferred that only the moisture transmis¬ sion control layer be completely penetrated by the perfora-
tions. Perforations are also referred to herein as "mesh- ings".
In a preferred embodiment, the perforations comprise a multiplicity or plurality of slits. The slits can be arranged in a wide variety of patterns. It is preferred that the slit pattern results in a non-expandable multi¬ layer membrane. Alternatively, the slit pattern can result in a multilayer membrane which expands upon the application of a lateral force to the multilayer membrane. An ex- pandable multilayer membrane can be stretched along at least one lateral axis. A "lateral axis" is an axis or line which traverses the entire cross section of a multi¬ layer membrane. When an expandable membrane is pulled in opposite directions at each end of the lateral axis, the membrane is stretched, thereby resulting in an increased surface area. An increase in surface area has the unde¬ sired effect of pulling the slits open to form permanent openings which will expose the underlying wound to in¬ creased moisture loss and external pathogens. Thus, it is essential to maintain the integrity of the membrane to optimize the proper function of the moisture control barri¬ er. Consequently, an expandable membrane is applied to a wound and maintained under conditions which prevent expan¬ sion or increases in the surface area of the membrane. A membrane which does not stretch or increase its surface area when pulled in opposite directions along a lateral axis is said to resist expansion along that lateral axis. A non-expandable multilayer membrane resists expan¬ sion along any lateral axis. A multilayer membrane having a multiplicity of slits resists expansion along a lateral axis when the lateral axis is not intersected by any slit. As a consequence, this type of multilayer membrane has a continuous band of membrane along the lateral axis that is free of slits. A non-expandable multilayer membrane has at least two perpen-
dicular continuous bands of membrane in which the slits do not intersect. As a result, the membrane can be pulled in opposite directions along any lateral axis without stretch¬ ing or increasing the surface area of the multilayer mem- brane.
One example of a multilayer membrane which resists expansion is shown in Figure 1. The slits are arranged in non-overlapping parallel rows with the slits in each row being parallel to one another. A continuous band of mem- brane in which the slits do not intersect runs the length of the membrane. Preferably, the slits are of equal length, are aligned with the slits in the same row, and are aligned with a slit in the adjacent row. Dimensions a, b and c in Figure 1 typically range from about 0.5 mm to about 5.0 mm in length. In one example, a is 1.7 mm, b is 1.1 mm and c is 2.7 mm.
A multilayer membrane is expandable along a first lateral axis when at least one slit intersects each lateral axis parallel to the first lateral axis. In a preferred example of an expandable multilayer membrane, the slits are arranged in non-overlapping parallel rows with the slits in each row being parallel to one another. The slits are of equal length and staggered with respect to the other slits in the row such that there is no continuous band of mem- brane between the rows which runs the length of the multi¬ layer membrane sample. As a result, the membrane will expand when opposing forces are applied at opposite ends of a lateral axis running perpendicular to the slits. This type of expandable membrane is shown in Figure 2. The degree of expansion is controlled by the length of the slits and the number of overlapping slits. Expandable membranes can be stretched, for example, to about 1.5, 2.0, 2.5 and 3.0 times their normal εurface area.
In another embodiment the perforations comprise a plurality of cross-slits. Cross-slits can open like a
valve, thereby maximizing fluid flow away from the wound site. As described above, the cross-slits can be arranged so that the membrane is expandable or is non-expandable. The cross-slits can be arranged into regular or irregular patterns, however regular patterns in which the slits are evenly spaced are preferred. Suitable patterns include polygonal patterns such trigonal, rectangular (see Figure 3) and hexagonal patterns.
In another embodiment the perforations comprise a plurality of holes. The holes are of sufficient size so that they do not clog, but are not so large that excessive amounts of fluid or water vapor escape from the wound. Suitable diameters of the holes are from about 100 microns to about 2 millimeters. As described for the cross-slits, holes can be arranged in irregular or regular patterns, however, regular patterns in which the holes are evenly spaced are preferred (see Figure 4; trigonal pattern shown) .
The perforated multilayer membranes and synthetic skins of the present invention are prepared by meshing conventional multilayer membranes and artificial skins with meshing machines such as a Collins a pligraft or a Brennen esher or a similar device used to prepare meshed auto¬ graft. A 1:1 meshing ratio is preferred. The same opera- tional technique to prepare meshed autograft may be used. Any other mechanical means for spacing the perforations in the patterns described herein can be used.
Multilayer membranes and synthetic skins are well known in the art and are disclosed in Yannas et al . , U.S. Patent No. 4,060,081, Yannas and Kirk, U.S. Patent No.
4,448,718 and Yannas et al. , U.S. Patent No. 4,947,840, the teachings of which are hereby incorporated into this appli¬ cation in their entirety. These and other multilayer membranes and synthetic skins suitable for perforation and use in the present invention are deεcribed below. It is to
be understood that there are many modifications which the skilled artisan could make to synthetic skin and multilay- ered membranes without affecting its suitability for use in the present invention. Many of these modifications are presently known in the art while others may be developed in the future. Such modifications are encompassed within the scope of the present invention. The multilayer membranes described herein have at least two layers of different materials. As illustrated in Figure 5, there is a first layer 10. Since the first layer 10 comes into direct contact with the subcutaneous tissue or wound bed, there are three essential characteristics required of this layer. These are: insolubility in body fluids; ability to promote the adherence and proliferation of cellε, such as fibro- blasts and endothelial cells; a controlled rate of biodeg- radation such that the material provides a scaffold suit¬ able for wound repair; and nonimmunogenicity. These multi¬ layer membranes also include at least one additional layer, which has the primary function of controlling the moisture flux for the overall membrane. Thus, moisture transmission control layer 12 is illustrated in Figure 5 as being di¬ rectly bonded to the first layer. It should be understood, however, that additional layers can be added on top of layer 12 or between first layer 10 and moisture control layer 12 as long as such additional layers do not interfere with the essential functions of layers 10 and 12.
The first layer is preferably a porous biodegradable polymeric membrane layer comprising a composite formed from collagen molecules that are crosslinked and covalently bonded with glycosa inoglycan (GAG) . Examples of specific glycosaminoglycans include but are not limited to chondroi- tin 6-sulfate, chondroitin 4-sulfate, heparan, heparan sulfate, keratan sulfate and dermatan sulfate. Also, other anionic polymers such as chitin and chitosan are suitable.
In a preferred embodiment of this invention, the average pore size of the biodegradable first layer is within the range of about 9 μm to about 630 μm, preferably about 20 μm to 200 μm. The average pore size can be calcu- lated by stereology from scanning electron micrograph of the surface or cross section as described by Dagalakis et al . J . of Biomedical Materials Research 14:511 (1980). Materials which do not come within these parameters do not delay or arrest skin wound contraction and thus tend to induce synthesis of undesirable scar tissue, while those materials having pore sizes within the desired upper and lower limits have been found to effectively delay or arrest skin wound contraction and induce synthesis of new func¬ tional tissue. Another determining factor in the effec- tiveness of multilayer membraneε is the pore volume frac¬ tion of the first layer. This value is defined as the percentage of the total volume of the material which is occupied by pore space. A more detailed definition is given in Fischmeister, H.F. Proceedings Int. Symp. RILEM/I- UPAC, Prague, September 18-21, 1973, Final Report Part II, p. C-439, the entire teachings of which are incorporated herein by reference. A high pore volume fraction in the first layer has been found to be clinically desirable, with pore volume fractions above about 80% being preferred. The degree of crosslink density is an important param¬ eter of this invention since it is a direct, controlling factor in the biodegradation rate of the material. Gener¬ ally, the greater the crosslink density, the lower the degradation rate, and vice versa. By controlling the degree of crosslinking, composites can be produced which exhibit a degradation rate within a range determined to be clinically desirable. The maximum degradation rate has been determined to be about 140 enzyme units (e.u.), and is preferably below about 120 e.u. The crosslinked composites should have an average molecular weight between crosslinks,
(Mc) , of between about 800 and about 60,000 daltons. Composites with an Mc of between 10,000 and about 40,000 tend to have the best balance between physical and thera¬ peutic properties and are this preferred. A preferred method for covalently crosslinking the collagen-GAG composites is known as aldehyde crosslinking. In this process, the materials are contacted with aqueous solutions of aldehyde, which serve to crosslink the materi¬ als. Suitable materials include formaldehyde, glutaralde- hyde and glyoxal. Glutaraldehyde is preferred because it yields the desired level of crosslink density more rapidly than other aldehydes and is also capable of increasing the crosslink density to a relatively high level. Composites suitable for use in the present invention can be made by forming an uncrosslinked material comprising a reaction product of collagen and a glycosaminoglycan and contacting the reaction product with an aqueous glutaraldehyde solu¬ tion for a period in excesε of one hour. The resulting crosslinked collagen-glycosaminoglycan composite has a rate of biodegradation which is low enough to enable the compos¬ ite to be a suitable scaffold for wound repair. The maxi¬ mum degradation rate has been determined to be about 140 enzyme units (e.u.), measured as described in Yannas et al . , U.S. Patent No. 4,947,840. Preferably, the biodegra- dation rate is below about 120 e.u.
It is preferred that the collagen quaternary structure after glutaraldehyde cross-linking be unbanded. Unbanded structures are characterized by the absence of periodic banding at 640A, characteristic of native collagen, when viewed by transmission electron microscopy (Sylvester et al . , Thrombosis Research 55:135 (1989)). Unbanded struc¬ tures can be obtained by crosslinking at pHs below about 4.25, preferably at about 3.0.
Covalent crosslinking can be achieved by other specif¬ ic techniques including radiation and dehydrother al meth¬ ods. An example of a suitable crosslinking technique is to treat collagen with 0.25% aqueous glutaraldehyde solu- tion in 0.05 M acetic acid for twenty four hours at 20- 25°C. These techniques are discussed in greater detail in Yannas et ai., U.S. Patent No. 4,060,081, Yannas and Kirk, U.S. Patent No. 4,448,718 and Yannas et al . , U.S. Patent No. 4,947,840, the entire teachings of which have been incorporated herein by reference. Other suitable chemical crosslinking techniques include carbodiimide coupling, azide coupling and diisocyanate crosslinking.
Particularly preferred first layer materials are crosslinked collagen-glycosaminoglycan composites contain- ing between about 6% and about 15% of a sulfate-containing mucopolysaccharide and crosslinked to an Mc value of be¬ tween about 5,000 and about 10,000. Chondroitin 6-sulfate forms especially outstanding composites.
A moisture transmission control layer is formed from a material which provides the moisture flux per unit area described above. These values are obtained by an appropri¬ ate combination of thickness, water transmission properties and the size, shape and pattern of the perforations. It is been found that a bilayer membrane produced as described in Example 1 and perforated through both the silicone and collagen GAG layer with a Brennen 1:1 mesher to give the slit pattern of Figure 1 showed no increase in vapor perme¬ ability (n=4) (0.65 + 0.04 mg/hr/cm2) compared with unper- forated material (0.64 +0.02 mg/hr/cm2). A bilayer mem- brane with perforations that cut the silicone layer, but only partially penetrated collagen-GAG, prepared with a modified Brennen mesher (1.236 inch diameter roller), operated with the silicone side of the membrane towards the
cutting blade, showed essentially the same vapor permeabil¬ ity (0.64 mg/hr/cm2).
The other essential property of this layer is that it be nontoxic. The material should contain no toxic sub- stances capable of diffusing out into tissues contacting a multilayer membrane graft or capable of being extracted therefrom. Also, the material should be capable of resist¬ ing enzymatic degradation or other degradation resulting from contact with other layers of the membrane or with tissue which degradation might lead to the production of substances that are toxic to neighboring tissue.
As is the case with the first layer, there are several other desirable properties for the layer which primarily controls the moisture flux. Thus, it is desirable that the moisture-control layer adhere to the wet surface of the first layer with a bond shear strength of at least about 10 psi, and preferably about 100 psi. It also is desirable that it have mechanical properties of: Young's modulus in the range of from about 100 to 1,000 psi; ultimate tensile strength of from about 100 to about 1,000 psi; and elonga¬ tion at break of from about 20 to about 100%
Additionally, it is advantageous if the moisture control layer is capable of being sterilized, i.e., of being subjected to physical or chemical treatment that kills bacteria and bacterial spores on its surface. Suit¬ able sterilization techniques include dry heat, exposure to ethylene oxide, irradiation, immersion in glutaraldehyde solution, etc.
Synthetic polymeric materials which can be used in the moisture control layer include: εilicone polymers, such as Silastic Medical Adhesive (Dow Corning) , a mixture of an hydroxyl terminated silicone polymer and methyl triethoxy silane which moisture-cures into a flexible, tough layer that adheres very well to first layer materials such as
crosslinked collagen-glycosaminoglycan composites; poly¬ acrylate or polymethacrylate esters or their copolymers such as an acrylic rubber latex formed from an ethyl acry- late-acrylic acid copolymer which forms a flexible film on top of first layer materials and which contains carboxylic acid groups capable of reacting with hydroxyl groups pres¬ ent in crosslinked collagen-glycosaminoglycan materials to form strong bonds; polyurethanes such as a reaction product of excess toluene diisocyante with a mixture of diols and triols to give a reactive, moisture-curing prepolymer capable of forming an elastomeric layer on crosslinked collagen-glycosaminoglycan composites and having chemical groups which react with amino groups or hydroxyl groups in such composites. Those skilled in the art will recognize or be able to ascertain, using no more than routine experi¬ mentation, other materials which are suitable for the moisture control layers.
Silicone polymer is preferred as the moisture control layer. It is available as a non-toxic product in a care- fully controlled medical grade. Its flow properties are of thixotropic nature, permitting uniform application by knife blade onto the surface of collagen-glycosaminoglycan com¬ posite layer with controlled penetration into the latter. Curing can be done at 100% relative humidity, thereby avoiding dehydration of the lower layer, consisting of the collagen-glycosaminoglycan composite, and preventing defor¬ mation of the multilayered structure. Silastic Medical Grade silicone typically has 180° peel strength, between 6 and 16 g/cm. Multilayer systems can also be made by using a mois¬ ture-curing silicone elastomer as the agent bonding the collagen-glycosaminoglycan layer to another material. By applying a thin film (1-2 thousandths of an inch) over a film prepared from εynthetic polymers such as the segmented polyurethanes, hydroxyethyl methacrylate and other "hydro-
gels", polyethylene terephthalate and polytetrafluoro¬ ethylene or from modified natural polymers such as cellu¬ lose acetate or from natural polymers such as elastin (the fibrous, insoluble, noncollagenous protein found in connec- tive tissue such as the thoracic aorta and ligamentum uchae) , a multicomponent composite can be obtained by curing at room temperature at 100% relative humidity for 16-24 hours.
If mechanical reinforcement is desired, a layer of gauze or other fabric or mesh could be usefully employed. Cotton or other textile mesh can be incorporated as a reinforcing mechanism by placing the textile material over the collagen-glycosaminoglycan composite and applying the Silastic silicone over the mesh onto the collagen-glycos- a inoglycan surface by knife coating. Curing at room temperature and 100% relative humidity overnight (16-24 hours) can result in a reinforced composite which is some¬ what stiffer than one without the mesh but with substan¬ tially improved tensile strength. The optimum thickness of a synthetic skin is related to the following parameters: (1) thickness of the skin to be replaced; (2) nature of wound and dimensions; (3) thickness of top layer required to control moisture flux; and, (4) relation of suturability and drapability to thick- ness.
The lowest attainable limit of thickness for the collagen-glycosaminoglycan layer is dependent upon the particle size of the collagen-glycosaminoglycan composites and is typically in the range of 1.5-2.0 mils. The upper limit of thickness depends only upon the application con¬ templated and in practice are available up to indefinitely high levels depending upon the quantity of dispersion filtered through a given area of filter. Thickness as high as 100-200 mils are readily prepared by the process de- scribed in U.S. Patent Nos. 4,060,081 and 4,947,840, al-
though for application as a skin substitute, the preferred range is 25-100 mils.
On the other hand, the thickness of the top layer would be dictated by the desired moisture flux, the mois- ture vapor transmission properties of the polymer used to form the top layer, and the need for the synthetic skin to be "drapable," i.e. to conform to the contour of the wound bed. In the case of Silastic Medical Grade silicone, a 5- mil thick silicone film layered onto a 50-mil thick layer of collagen-mucopolysaccharide composite is a typical multilayer artificial skin having the desired range of moisture flux. A preferred range for the thickness of the silicone layer is 4-mil to 15-mil, which provides moisture permeability in the range of 0.1 to 2 mg/hr/cm2, sufficient mechanical strength to allow suturing or stapling and is drapable to allow conformation to wound beds.
The multilayer membranes described herein are useful as dresεings for the treatment of burns, cuts, lacerations, abrasions and other such conditions which involve injury or destruction of skin by mechanical, thermal, chemical or other external insult by local or systemic disease. Also, the membranes themselves can be used as artificial grafts wherein they temporarily replace functions of normal skin and provide a template for permanent cellular regeneration. The invention is further and more specifically illus¬ trated by the following examples.
Example 1 - Preparation of the Artificial Skin
Artificial skin was produced according to the proce¬ dure described in Yannas et al . , U.S. Patent No. 4,947,840. The primary modifications to this procedure, aside from scaling up the size of the batch, are the use of bovine tendon collagen rather than bovine hide collagen, and that
black threads were embedded in the silicone layer for ease of identification.
Example 2 - Efficacy Of Perforated Artificial Skin Versus Unperforated Artificial Skin
Patients Chosen for the Study
Twenty patients were enrolled in the study. All patients were required to complete a minimum of 12 months of study following healing of the epidermal autograft, as well as to have procedurally correct case reports. Eligibility was determined in part by age and burn size. Patients of any age up to age 70 were eligible for entry into the study according to the following sliding scale:
AGE BURN SIZE less than 50 years Any burn Size
50-59 years 40% or less TBSA
60-69 years 30% or less TBSA
70 years and older Not Eligible TBSA is the total body surface area
Males and nonpregnant females were allowed entry into this study. In addition, patients were chosen who had thermal burn injuries which were, as judged by the investi¬ gator, deep partial-thickness or full-thickness wounds and amenable to excisional therapy, had been hospitalized within 48 hours of burn injury and in whom excision of eschar started within 7 days of burn injury and completed within 21 days after the injury.
Patients who had significant concomitant disease, electrical or chemical burns, were pregnant, had wounds infected to a clinically significant degree, or had wounds previously treated by excisional therapy were excluded from the study.
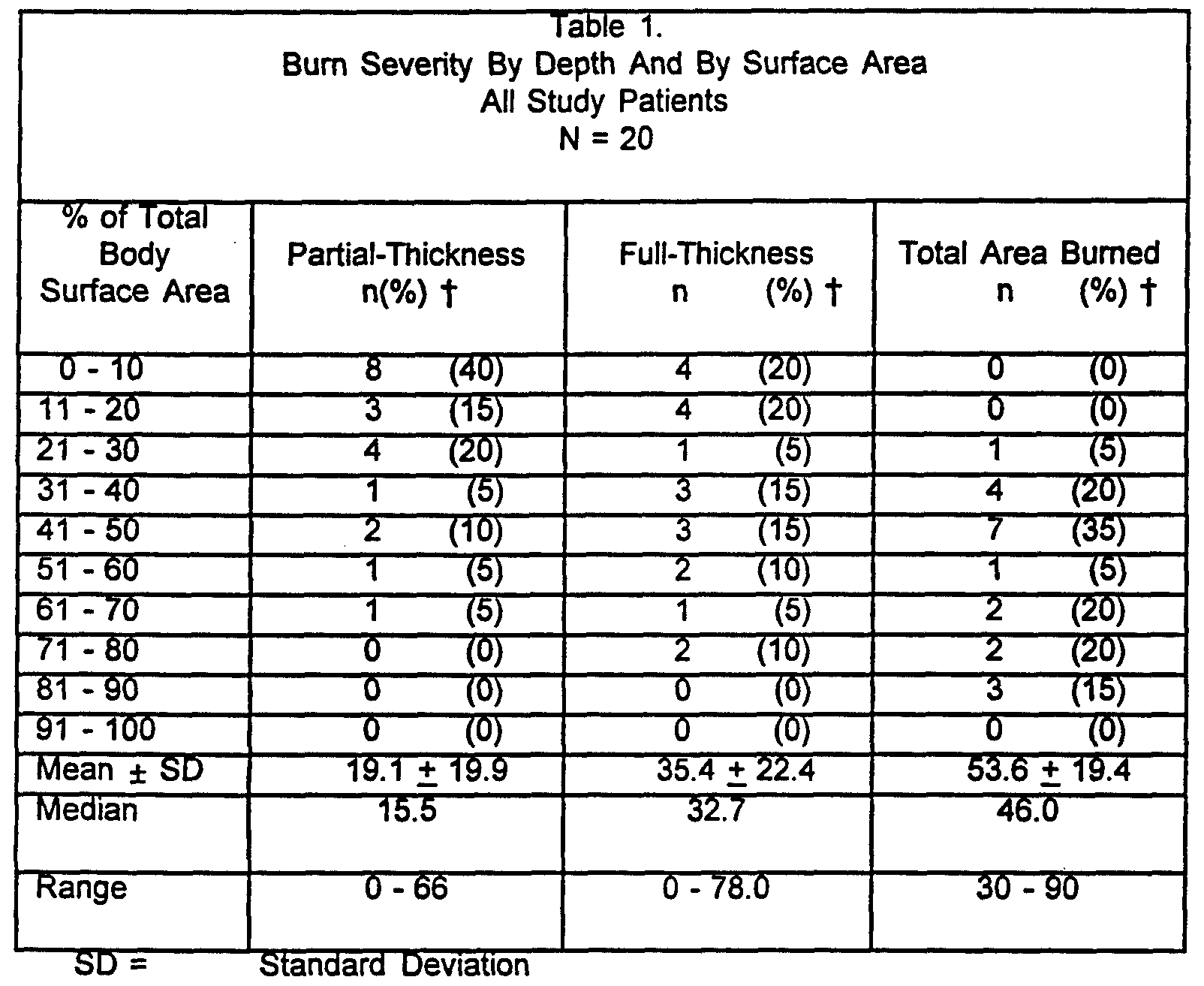
Application of the perforated artificial skin was begun within seven days of injury and completed within 21 days of injury. Patients entered this clinical trial with full-thickness or deep partial-thickness injuries requiring excision. Table 1 describes the percentage of total body surface area (TBSA) and depth (partial-thickness, full- thickness and total) of the wound for all patients.
t = % TBSA data by row do not sum to 100%, reflecting the distribution of TBSA injury across all patients
Procedure for Applying the Artificial Skin
The artificial skin of Example 1 was thoroughly rinsed in sterile normal saline prior to application. The rinsing
procedure was to soak one of the artificial skin devices in sterile, pyrogen free, normal saline solution for 10 min¬ utes, changing the solution twice.
For some wound sites the artificial skin was cut with a Collins ampligraft with a 2:1 meshing ratio, similar to Figure 2, to give a slitted artificial skin capable of being expanded to cover twice its normal area. In this study, however, the artificial skin was not expanded. The wound was then excised to the level of viable tissue. The excisional techniques used for the artificial skin sites were fascial, sequential, or tangential. It is critical to the succeεεful take of the artificial skin that excision be complete and that no eschar remain.
Complete hemostasis was also achieved before applica- tion of the artificial skin by fine needle point cauteriza¬ tion and application of topical epinephrine at concentra¬ tion of 1:10,000. The presence of hematoma will cause loss of the artificial skin take in the affected area. Broad area cauterization that could decrease wound bed viability was avoided.
The artificial skin was accurately shaped to fit the excised wound margins to minimize scarring at these mar¬ gins; it was not allowed to overlap onto nonexcised areas or onto other sheets of the artificial skin. The artifi- cial skin was cut with sterile scissors by placing the sheet of artificial skin over the open area and cutting it exactly to the edge of the wound.
The artificial skin was applied to the wound so that the collagen template layer was in direct contact with the excised wound. The silicone layer (identified by the black threads) was placed out (away from the wound bed) . The material readily adhered and conformed to the wound sur¬ face. Any air bubbles were carefully removed by moving them to the edge of the sheet. The artificial skin εheets were secured by staples or sutures placed in an interrupted
fashion (with fine synthetic monofilament suture, or 4/0 or 5/0 chromic, using a fine atraumatic needle) under slight tension. Care was taken not to spread or expand the mem¬ brane and to achieve a primary closure between the artifi- cial skin and adjacent unburned skin or between sheets of the artificial skin. Each strip of artificial skin was sutured or stapled in place independently.
The area was covered with an inner dressing consisting of a single layer of wide mesh gauze, secured by staples or sutures to the normal tissue at the edges of the grafted area. This layer was then wrapped with an outer dressing consisting of two or three layers of 4 inch (10.2 cm) wide rolled gauze.
Postoperative Care The postoperative care followed a similar protocol to that used following treatment with full sheet or meshed autograft. Dressings were inspected daily for evidence of infection. The patient was also monitored for evidence of sepsis. The outer dressing were changed every 4 to 5 days. The inner dressing was not disturbed unless there were problems requiring intervention. The attachment of the silicone layer was also examined. Fluid accumulation was treated by excising the silicone layer over the affected area.
The silicone layer was removed after the collagen layer had been replaced by neodermis, usually 14-21 days after grafting using forceps. After removal of the sili¬ cone layer, a thin layer of meshed epidermal autograft (0.002 to 0.005 inches) was applied to the artificial skin neodermis by conventional techniques.
Statistical Methodology
Descriptive statistics are given for all entry, treat¬ ment, and outcome characteristics. Frequencies and confi¬ dence intervals were used to summarize the infection and culture results. Percentages were used to summarize physi¬ cian assessments at each follow-up visit. Confidence intervals for dichotomous data were computed using the binomial distribution. The method of Bickel and Doksum, Mathematical Statistics - Basic Ideas and Selected Topic, Holden-Day, San Francisco, 180-2 (1977) , was used for the calculations. Confidence intervals for continuous data were computed for both the mean and the median. Confidence intervals for the median were based on the Sign Test (Hollander and Wolfe, Nonparametric Statistical Meth- ods , Wiley, New York, 48-9 (1976)).
Because of the skewed nature of the artificial skin take and epidermal-autograft take variables, nonparametric statistics were used to analyze effect on take in all comparisons. The Kruskal-Walliε test SAS/STAT User's Guide, SAS Institute, Inc., Cary, North Carolina 27513, was used to test for differences in both the artificial skin take and epidermal-autograft take among anatomic locations. The Kruskal-Walliε test was also used to test for significance of day of excision on the artificial skin take. The maximum-likelihood, chi-square test waε used to test for differences in poor take of the artificial skin (<10%) among anatomic locations.
Results
Table 2 gives the distribution of the artificial skin take over the 56 wound sites for which take was fully evaluated. Overall the take of the artificial skin to freshly excised burn injuries was greater than 80% in 41 of the 56 (73.2%) wound sites. Twenty-nine (52%) of the wound sites had 100% artificial skin take. Conversely, 8 of the
56 (14%) wound siteε had 10% or less take. Five wound sites (9%) had 0% take. For all sites in total the mean take was 80.6% and the median take was 100%.
When comparing the perforated artificial skin to the sheet artificial skin, perforated artificial skin had greater than 80% take in 28 of the 34 (82%) wound sites. Sites receiving sheet artificial skin had greater than 80% take in only 13 of the 22 (59%) wound sites studied. Twenty sites (59%) treated with perforated artificial skin had 100% take, whereas nine sites (41%) that received full sheet artificial skin had 100% take. Both the full sheet and perforated artificial εkin had 0% take in 9% of the sites evaluated. The mean take for perforated and full sheet were 85% and 74%, reεpectively. The medians were 100% for perforated and 95% for full sheet artificial skin.
SD = Standard Deviation
Sixteen of 56 (29%) were reported as having fluid accumulation under the artificial skin. Ten of 22 sheet sites (46%) had fluid accumulation reported, but only 6 of 34 meshed sites (18%) had fluid accumulation. Table 3 indicates the assessment of cultures taken on the day of epidermal autografting and the assessment of clinical infection based on those culture results for the 36 sites with culture data on that day. For the pooled perforated and sheet artificial skin data, positive cul- tures were found in 30 wound sites (83%) . Two (6%) were reported as clinically significant. Fifteen (75%) of the perforated artificial skin sites had positive cultures immediately postexcision, none of the perforated artificial skin sites were reported to be clinically significant. Fifteen (94%) of the sheet artificial sites had positive cultures immediately postexcision, two sites (12%) were reported to be clinically significant. The perforated artificial skin appears to have a lower percentage of positive cultures than the sheet artificial skin. Also, none of the perforated artificial skin were reported to have significant clinical infections, whereas two sheet artificial sites were reported to have clinical infections.
Table 3
Cultured Results on the Day of Epidermal Autograft
All Study Patients N = 20
Meshed Sheet Total N = 22 sites N = 16 sites N = 38 sites
Source Freq (%) Freq (%) Freq (%) 95% Cl
Positive 15 (75) 15 (94) 30 (83) (72, 95) Culture
Clinically 0 (0) 2 (12) 2 (6) (0, 14) Significant
Cl = Confidence Interval
References: Supporting Data for the Meshed vs. Sheet
INTEGRA Study, Data Listings 4A, page 06-0213 and 7B, page
06-0281
Wounds considered to be suspiciously infected by visual observation were also cultured. Table 4 shows the results of cultures obtained after the day of excision and before the day of the epidermal autograft. If there were repeated observations on a single day, only the last deter¬ mination was used in this analysis. There were 98 addi¬ tional cultures obtained after the day of excision and before the day of epidermal autograft used in the analysis. Of those, 88 (90%) were positive cultures and 33 (34%) were considered clinically significant (Table 4) . The meshed artificial skin appeared to have a lower percentage of sites with positive cultureε (85% vs. 94% for sheet sites) as well as a lower percentage of siteε with clinically significant culture results (14% vs. 55%) .
Table 4
Cultured Results After Excision and Before Epidermal Autograft All Study Patients N = 20
Meshed Sheet Total N = 51 sites N = 47 sites N = 98 sites
Source Freq (%) Freq (%) Freq (%) 95% Cl
Positive 44 (86) 44 (94) 88 (90) (84, 96) Culture
Clinically 7 (14) 26 (55) 33 (34) (25, 43) Significant
Cl = Confidence Interval
Eguivalents
Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, many equivalents to the specific embodiments of the invention described specifically herein. Such equivalents are in¬ tended to be encompassed in the scope of the following claims.
Claims
1. A method of preventing or reducing infection at a wound site (e.g., a burn wound) which is undergoing repair with a synthetic skin graft (e.g., a porous biodegradable polymeric multilayer membrane) compris¬ ing providing a moisture control layer disposed on the skin graft which is perforated such that when the bottom layer of the skin graft is applied to a wound, the moisture control layer is permeable to fluid in the presence of hydrostatic presεure from exudate in the wound and εubstantially impermeable to fluid and water vapor in the wound in the absence of hydrostatic pressure from exudate in the wound.
2. A method of covering a burn or wound site on a human or animal, comprising applying to a burn or wound a multilayer membrane (e.g., two layerε) comprising a porous biodegradable polymeric membrane layer having disposed thereon a moisture control layer that is perforated such that when the bottom layer of the multilayer membrane is applied to a wound, the mem¬ brane is permeable to fluid in the presence of hydro¬ static pressure from exudate in the wound and substan¬ tially impermeable to fluid and water vapor in the wound in the absence of hydrostatic preεεure from exudate in the wound.
3. A multilayer membrane, compriεing a porous biodegrad¬ able polymeric membrane layer having disposed thereon a moisture control layer that is perforated such that when the bottom layer of the multilayer membrane (e.g. , two layers) is applied to a wound, the membrane is permeable to fluid in the presence of hydrostatic pressure from exudate in the wound and subεtantially substantially impermeable to fluid and water vapor in the wound in the absence of hydrostatic pressure from exudate in the wound.
4. Synthetic skin, comprising a porous biodegradable polymeric membrane layer having disposed thereon a moisture control layer that is perforated such that when the bottom layer of the synthetic skin is applied to a wound, the synthetic skin is permeable to fluid in the presence of hydrostatic presεure from exudate in the wound and εubstantially impermeable to fluid and water vapor in the wound in the absence of hydrostatic presεure from exudate in the wound.
5. The multilayer membrane, synthetic skin or method according to any one of Claims 1 to 4 wherein the polymeric layer has a pore size from between about 9 μm and about 630 μm (e.g., between about 20 μm and about 200 μm) and a pore volume fraction of greater than about 80%.
6. The multilayer membrane, synthetic skin or method according to any one of Claims l to 5 wherein the moisture control layer is perforated so that moisture loss from a wound to which the bottom layer of the multilayer membrane has been applied iε maintained below about 2.2 mg/cm2/hour.
7. The multilayer membrane, synthetic skin or method according to any one of Claims 1 to 6 wherein the polymeric layer compriseε crosslinked collagen, a crosεlinked collagen-glycosaminoglycan composite, or unbanded collagen-glycosaminoglycan.
8. The multilayer membrane, synthetic skin or method according to any one of Claims l to 7 wherein the moisture control layer is formed from a synthetic polymer selected from the group consisting of silicone resins, polyurethane, polyacrylate esters, polymethacrylate esters and polyurethanes.
9. The multilayer membrane, synthetic skin or method according to any one of Claims 1 to 8 wherein the perforations additionally penetrate the polymeric membrane layer or the skin graft.
10. The multilayer membrane, synthetic skin or method according to any one of Claims 1 to 9 wherein (i) the perforationε comprise a plurality of slits (e.g., the slits are parallel and arranged in aligned or staggered rows) , (ii) the perforations comprise a plurality of cross slits arranged in parallel rows, or (iii) the perforations comprise a plurality of holes arranged in a trigonal pattern.
11. The multilayer membrane, synthetic skin or method of Claim 10 wherein the moisture control layer has (i) at least two perpendicular continuous bands of membrane which the slits do not intersect, thereby preventing expansion of the membrane upon application of a force along the continuous bandε of membrane, (ii) parallel, lateral axiε εuch that at leaεt one slit intersectε each parallel, lateral axiε, thereby allowing expansion of the membrane upon application of a force along the lateral axis, or (iii) a sufficient number of slits intersects each parallel lateral axis such that the membrane can expand to about twice its original area upon application of force along the lateral axiε.
12. A multilayer membrane, synthetic skin or method according to any one of Claims 1 to 12 comprising a polymeric membrane layer which: (1) has controllable degradability in the presence of body enzymes; (2) has controllable solubility in the presence of body fluids; (3) is εubstantially nonimmunogenic upon grafting or implantation; (4) provokes no substantial foreign body response upon grafting or implantation; (5) and, promotes the adherence of cells, such as fibroblasts and endothelial cells.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU67755/96A AU6775596A (en) | 1995-08-16 | 1996-08-15 | Perforated artificial skin grafts |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US244295P | 1995-08-16 | 1995-08-16 | |
| US60/002,442 | 1995-08-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1997006837A1 true WO1997006837A1 (en) | 1997-02-27 |
Family
ID=21700783
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1996/013244 WO1997006837A1 (en) | 1995-08-16 | 1996-08-15 | Perforated artificial skin grafts |
Country Status (2)
| Country | Link |
|---|---|
| AU (1) | AU6775596A (en) |
| WO (1) | WO1997006837A1 (en) |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998013076A1 (en) * | 1996-09-24 | 1998-04-02 | Brigham And Women's Hospital | Cultured cell-seeded fibrous lattice for tissue replacement |
| WO1999000151A2 (en) * | 1997-06-26 | 1999-01-07 | Smith & Nephew Plc | Cell culture products |
| US6773723B1 (en) | 2000-08-30 | 2004-08-10 | Depuy Acromed, Inc. | Collagen/polysaccharide bilayer matrix |
| WO2009093023A3 (en) * | 2008-01-25 | 2010-06-24 | Smith & Nephew Plc | Multilayer scaffold |
| US8049059B2 (en) * | 2001-10-26 | 2011-11-01 | Cook Biotech Incorporated | Medical graft device with meshed structure |
| WO2013050429A1 (en) * | 2011-10-07 | 2013-04-11 | Neotherix Limited | Sampling device |
| WO2014138309A1 (en) * | 2013-03-06 | 2014-09-12 | Awod, Inc., A California Corporation | Chronic wound dressing with variable pore sizes |
| EP3020410A1 (en) | 2008-04-18 | 2016-05-18 | Collplant Ltd. | Methods of generating and using procollagen |
| WO2017044682A1 (en) * | 2015-09-11 | 2017-03-16 | Lifecell Corporation | Perforated tissue matrix |
| WO2018165131A1 (en) * | 2017-03-06 | 2018-09-13 | Tei Biosciences, Inc. | Perforated tissue graft |
| WO2020150382A3 (en) * | 2019-01-15 | 2020-09-10 | Schultz Brent | Compliant biological scaffold |
| CN111700713A (en) * | 2020-06-24 | 2020-09-25 | 福建华民生物科技有限公司 | Artificial skin |
| US10869745B2 (en) | 2016-10-06 | 2020-12-22 | Lifecell Corporation | Tissue matrix with preformed openings or pilot openings |
| EP3315145B1 (en) | 2016-10-28 | 2022-06-08 | BSN medical GmbH | Multi-layer wound care product with perforated collagen layer |
| WO2023058061A1 (en) * | 2021-10-07 | 2023-04-13 | Pacify Medical Technologies Private Limited | A skin mincing device |
| US11925532B2 (en) | 2021-12-10 | 2024-03-12 | Vivex Biologics Group, Inc. | Vented wound dressing barrier |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4060081A (en) * | 1975-07-15 | 1977-11-29 | Massachusetts Institute Of Technology | Multilayer membrane useful as synthetic skin |
| EP0399782A2 (en) * | 1989-05-23 | 1990-11-28 | Minnesota Mining And Manufacturing Company | Modifying a membrane for use as a graft |
| EP0462426A1 (en) * | 1990-06-01 | 1991-12-27 | FIDIA S.p.A. | Biocompatible perforated membranes and their use as artificial skin |
| US5489304A (en) * | 1994-04-19 | 1996-02-06 | Brigham & Women's Hospital | Method of skin regeneration using a collagen-glycosaminoglycan matrix and cultured epithelial autograft |
-
1996
- 1996-08-15 WO PCT/US1996/013244 patent/WO1997006837A1/en active Application Filing
- 1996-08-15 AU AU67755/96A patent/AU6775596A/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4060081A (en) * | 1975-07-15 | 1977-11-29 | Massachusetts Institute Of Technology | Multilayer membrane useful as synthetic skin |
| EP0399782A2 (en) * | 1989-05-23 | 1990-11-28 | Minnesota Mining And Manufacturing Company | Modifying a membrane for use as a graft |
| EP0462426A1 (en) * | 1990-06-01 | 1991-12-27 | FIDIA S.p.A. | Biocompatible perforated membranes and their use as artificial skin |
| US5489304A (en) * | 1994-04-19 | 1996-02-06 | Brigham & Women's Hospital | Method of skin regeneration using a collagen-glycosaminoglycan matrix and cultured epithelial autograft |
Non-Patent Citations (1)
| Title |
|---|
| S.T. BOYCE ET AL.: "BIOLOGIC ATTACHMENT, GROWTH, AND DIFFERENTIATION OF CULTURED HUMAN EPIDERMAL KERATINOCYTES ON A GRAFTABLE COLLAGEN AND CHONDROITIN-6-SULFATE SUBSTRATE", SURGERY, vol. 103, no. 4, April 1988 (1988-04-01), pages 421 - 431, XP002023073 * |
Cited By (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998013076A1 (en) * | 1996-09-24 | 1998-04-02 | Brigham And Women's Hospital | Cultured cell-seeded fibrous lattice for tissue replacement |
| US6800282B1 (en) * | 1997-06-26 | 2004-10-05 | Smith & Nephew, Plc | Cell culture products |
| WO1999000151A2 (en) * | 1997-06-26 | 1999-01-07 | Smith & Nephew Plc | Cell culture products |
| WO1999000151A3 (en) * | 1997-06-26 | 1999-03-25 | Smith & Nephew | Cell culture products |
| US6936276B2 (en) | 2000-08-30 | 2005-08-30 | Depuy Acromed, Inc. | Collagen/polysaccharide bilayer matrix |
| US6896904B2 (en) | 2000-08-30 | 2005-05-24 | Depuy Spine, Inc. | Collagen/polysaccharide bilayer matrix |
| US6773723B1 (en) | 2000-08-30 | 2004-08-10 | Depuy Acromed, Inc. | Collagen/polysaccharide bilayer matrix |
| US6939562B2 (en) | 2000-08-30 | 2005-09-06 | Depuy Acromed, Inc. | Collagen/polysaccharide bilayer matrix |
| US8049059B2 (en) * | 2001-10-26 | 2011-11-01 | Cook Biotech Incorporated | Medical graft device with meshed structure |
| WO2009093023A3 (en) * | 2008-01-25 | 2010-06-24 | Smith & Nephew Plc | Multilayer scaffold |
| EP3020410A1 (en) | 2008-04-18 | 2016-05-18 | Collplant Ltd. | Methods of generating and using procollagen |
| WO2013050429A1 (en) * | 2011-10-07 | 2013-04-11 | Neotherix Limited | Sampling device |
| US10687790B2 (en) | 2011-10-07 | 2020-06-23 | Neotherix Limited | Sampling device |
| WO2014138309A1 (en) * | 2013-03-06 | 2014-09-12 | Awod, Inc., A California Corporation | Chronic wound dressing with variable pore sizes |
| CN107949345A (en) * | 2015-09-11 | 2018-04-20 | 生命细胞公司 | The periplast of perforation |
| US11383007B2 (en) | 2015-09-11 | 2022-07-12 | Lifecell Corporation | Perforated tissue matrix |
| US10537665B2 (en) | 2015-09-11 | 2020-01-21 | Lifecell Corporation | Perforated tissue matrix |
| WO2017044682A1 (en) * | 2015-09-11 | 2017-03-16 | Lifecell Corporation | Perforated tissue matrix |
| RU2728627C2 (en) * | 2015-09-11 | 2020-07-30 | Лайфселл Корпорейшн | Perforated fabric matrix |
| JP2018526148A (en) * | 2015-09-11 | 2018-09-13 | ライフセル コーポレーションLifeCell Corporation | Perforated tissue matrix |
| CN112972062A (en) * | 2015-09-11 | 2021-06-18 | 生命细胞公司 | Perforated tissue matrix |
| US10869745B2 (en) | 2016-10-06 | 2020-12-22 | Lifecell Corporation | Tissue matrix with preformed openings or pilot openings |
| EP3315145B1 (en) | 2016-10-28 | 2022-06-08 | BSN medical GmbH | Multi-layer wound care product with perforated collagen layer |
| WO2018165131A1 (en) * | 2017-03-06 | 2018-09-13 | Tei Biosciences, Inc. | Perforated tissue graft |
| US11633520B2 (en) | 2017-03-06 | 2023-04-25 | Tei Biosciences, Inc. | Perforated tissue graft |
| US20230263940A1 (en) * | 2017-03-06 | 2023-08-24 | Tei Biosciences, Inc. | Perforated tissue graft |
| WO2020150382A3 (en) * | 2019-01-15 | 2020-09-10 | Schultz Brent | Compliant biological scaffold |
| CN111700713A (en) * | 2020-06-24 | 2020-09-25 | 福建华民生物科技有限公司 | Artificial skin |
| WO2023058061A1 (en) * | 2021-10-07 | 2023-04-13 | Pacify Medical Technologies Private Limited | A skin mincing device |
| US11925532B2 (en) | 2021-12-10 | 2024-03-12 | Vivex Biologics Group, Inc. | Vented wound dressing barrier |
Also Published As
| Publication number | Publication date |
|---|---|
| AU6775596A (en) | 1997-03-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO1997006837A1 (en) | Perforated artificial skin grafts | |
| KR100860896B1 (en) | Bioabsorbable Wound Dressing | |
| US4051848A (en) | Synthetic skin wound dressing | |
| US4060081A (en) | Multilayer membrane useful as synthetic skin | |
| AU2011256404B2 (en) | Reinforced absorbable multi-layered fabric for hemostatic applications | |
| US3903882A (en) | Composite dressing | |
| CN101132817B (en) | Multilayer bandage using for wound healing | |
| EP0637452B1 (en) | Collagen membrane material for medical use and process for preparing the same | |
| US5607590A (en) | Material for medical use and process for preparing same | |
| US3896802A (en) | Flexible flocked dressing | |
| JP3799626B2 (en) | Cardiovascular repair material and method for producing the same | |
| WO2017148255A1 (en) | Composite soft tissue repairing material for stabilizing repair region | |
| US20040018227A1 (en) | Multilayered microporous foam dressing and method for manufacturing the same | |
| US7932429B2 (en) | Device designed for regenerating the human dermis and process for producing said device | |
| EP0734736A1 (en) | Medical device and method for producing the same | |
| WO1998022157A1 (en) | Collagen material and process for producing the same | |
| Beumer et al. | A new biodegradable matrix as part of a cell seeded skin substitute for the treatment of deep skin defects: a physico-chemical characterisation | |
| Mao et al. | Nonwoven wound dressings | |
| WO1997017044A1 (en) | Membrane for skin removed wound | |
| JPH04108444A (en) | Artificial skin | |
| US20040002772A1 (en) | Tissue equivalents with perforations for improved integration to host tissues and methods for producing perforated tissue equivalents | |
| JP2000245450A (en) | Sheet for cultured skin improving taking rate | |
| JPH0481466B2 (en) | ||
| JPH01104257A (en) | Artificial skin and its preparation | |
| JPH0234165A (en) | Artificial skin and manufacture |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AU CA JP |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LU MC NL PT SE |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: CA |