WO2011133584A2 - Nucleic acid compounds for inhibiting hras gene expression and uses thereof - Google Patents
Nucleic acid compounds for inhibiting hras gene expression and uses thereof Download PDFInfo
- Publication number
- WO2011133584A2 WO2011133584A2 PCT/US2011/033102 US2011033102W WO2011133584A2 WO 2011133584 A2 WO2011133584 A2 WO 2011133584A2 US 2011033102 W US2011033102 W US 2011033102W WO 2011133584 A2 WO2011133584 A2 WO 2011133584A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- nucleic acid
- antisense strand
- hras
- rna
- seq
- Prior art date
Links
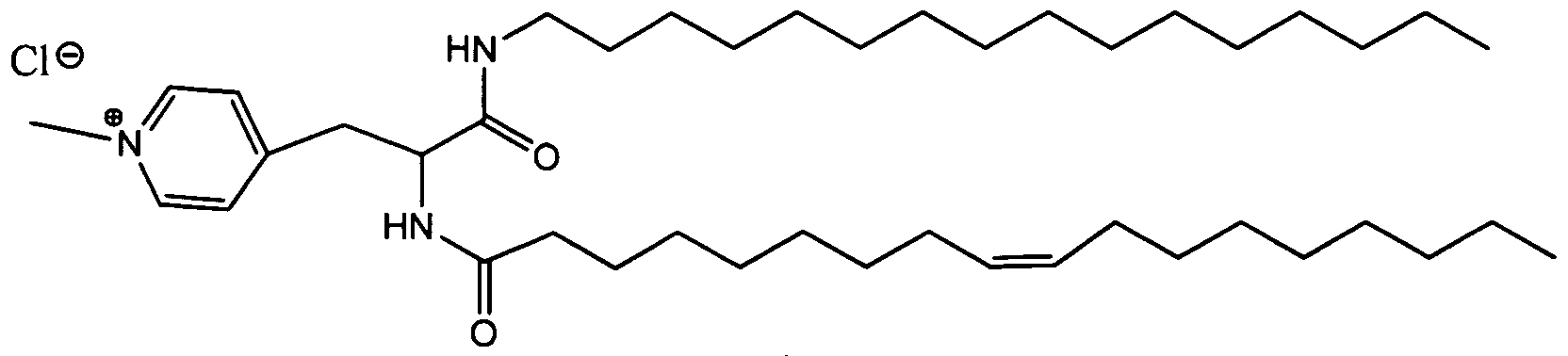
- MXZJVSYHTBEAFZ-XUTLUUPISA-N CCCCCCCCCCCCCCCCNC(C(CCNC(N(C)C)=N)NC(CCCCCCC/C=C/CCCCCCCC)=O)=O Chemical compound CCCCCCCCCCCCCCCCNC(C(CCNC(N(C)C)=N)NC(CCCCCCC/C=C/CCCCCCCC)=O)=O MXZJVSYHTBEAFZ-XUTLUUPISA-N 0.000 description 1
- NAHKRAAVZKZGEP-CZIZESTLSA-N CCCCCCCCCCCCCCCCNC(C(CCNC(NC)=N)NC(CCCCCCC/C=C/CCCCCCCC)=O)=O Chemical compound CCCCCCCCCCCCCCCCNC(C(CCNC(NC)=N)NC(CCCCCCC/C=C/CCCCCCCC)=O)=O NAHKRAAVZKZGEP-CZIZESTLSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1135—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against oncogenes or tumor suppressor genes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering N.A.
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/321—2'-O-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/344—Position-specific modifications, e.g. on every purine, at the 3'-end
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Organic Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Zoology (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Plant Pathology (AREA)
- Veterinary Medicine (AREA)
- Microbiology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Biochemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
This disclosure provides double- stranded nucleic acid complexes having one or more hydroxymethyl substituted nucleomonomer(s) and wherein one strand is complementary to a HRAS mRNA. Nucleic acid complexes of the disclosure may be useful for therapeutic applications, diagnostic applications or research applications. Nucleic complexes include short interfering RNA complexes (siRNA) capable of modulating gene expression comprising an antisense strand and a continuous or a discontinuous passenger strand ("sense strand"). Further, one or more hydroxymethyl substituted nucleomonomer(s) of this disclosure may be positioned at the 3 '-end, at the 5 '-end, at both the 3 '-end and 5 'end, and/or in a double-stranded region of a nucleic acid complex. Also provided are methods of decreasing expression of a HRAS gene in a cell or in a subject to treat a HRAS-related disease.
Description
NUCLEIC ACID COMPOUNDS FOR INHIBITING HRAS GENE EXPRESSION AND USES THEREOF
TECHNICAL FIELD
The present disclosure relates generally to compounds for use in treating disease by gene silencing and, more specifically, to a double-stranded nucleic acid complexes comprising an antisense strand and a continuous passenger strand or a discontinuous passenger strand ("sense strand") that decreases expression of a RAS viral (v-ras) oncogene homolog (RAS) mRNA, for example v-Ha-ras Harvey rat sarcoma viral oncogene homolog gene (HRAS), and to uses of such nucleic acid complexes to treat or prevent cancer associated with inappropriate HRAS gene expression. Further, the disclosure provides double-stranded nucleic acid complexes having one or more hydroxymethyl substituted nucleomonomer(s) and wherein one strand is complementary to a HRAS mRNA.
BACKGROUND
RNA interference (RNAi) refers to the cellular process of sequence specific, post-transcriptional gene silencing in animals mediated by small inhibitory nucleic acid molecules, such as a double-stranded RNA (dsRNA) that is homologous to a portion of a targeted messenger RNA (Fire et al., Nature 397 :806, 1998; Hamilton et al., Science 286:950- 951 , 1999). RNAi has been observed in a variety of organisms, including mammalians (Fire et al., Nature 397:806, 1998; Bahramian and Zarbl, Mol. Cell. Biol. 79:274-283, 1999; Wianny and Goetz, Nature Cell Biol. 2:70, 1999). RNAi can be induced by introducing an exogenous synthetic 21 -nucleotide RNA duplex into cultured mammalian cells (Elbashir et al., Nature 411:494, 2001a).
The mechanism by which dsRNA mediates targeted gene-silencing can be described as involving two steps. The first step involves degradation of long dsRNAs by a ribonuclease III- like enzyme, referred to as Dicer, into short interfering RNAs (siRNAs) having from 21 to 23 nucleotides with double-stranded regions of about 19 base pairs and a two nucleotide, generally, overhang at each 3'-end (Berstein et al., Nature 409:363, 2001 ; Elbashir et al., Genes Dev. 75: 188, 2001b; and Kim et al., Nature Biotech. 23:222, 2005). The second step of RNAi gene-silencing involves activation of a multi-component nuclease having one strand (guide or antisense strand) from the siRNA and an Argonaute protein to form an RNA-induced silencing complex ("RISC") (Elbashir et al., Genes Dev. 75: 188, 2001). Argonaute initially associates with a double-stranded siRNA and then endonucleolytically cleaves the non-incorporated strand
(passenger or sense strand) to facilitate its release due to resulting thermodynamic instability of the cleaved duplex (Leuschner et al., EMBO 7:314, 2006). The guide strand in the activated RISC binds to a complementary target mRNA, which is then cleaved by the RISC to promote gene silencing. Cleavage of the target RNA occurs in the middle of the target region that is complementary to the guide strand (Elbashir et al., 2001b).
There continues to be a need for alternative effective therapeutic modalities useful for treating or preventing HRAS-associated diseases or disorders in which reduced HRAS gene expression (gene silencing) would be beneficial. The present disclosure meets such needs, and further provides other related advantages. BRIEF SUMMARY
Briefly, the present disclosure provides double-stranded RNA (dsRNA) comprising a continuous strand or a discontinuous sense strand, and an antisense strand that is suitable as a substrate for Dicer or as a RISC activator to modify expression of HRAS messenger RNA (mRNA). Further, the disclosure provides double- stranded nucleic acid complexes having one or more hydroxymethyl substituted nucleomonomer(s) and wherein one strand is complementary to a HRAS mRNA.
In one aspect, the instant disclosure provides a nucleic acid that down regulates the expression of a v-Ha-ras Harvey rat sarcoma viral oncogene homolog gene (HRAS) mRNA, the nucleic acid comprising an antisense strand having a region of 15 to 60 (or 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24 ,25 ,26, 27, 28, 29, 30, 31 , 32, 33, 34 ,35 ,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 , 52, 53, 54, 55, 56, 57, 58, 59, or 60) contiguous nucleomonomers, wherein at least 15 contiguous nucleomonomers of the nucleic acid correspond to 15 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81 , 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278, and a sense strand complementary to the antisense strand, wherein the antisense strand and the sense strand can anneal to form a double-stranded region of 15 base pairs to 60 base pairs. In certain embodiments, the nucleic acid is a ribonucleic acid having a double-stranded region (dsRNA). In certain embodiments, the ribonucleic acid is a siRNA.
In other embodiments, the antisense strand is 18, 19, 20, 21 , 22, 23, 24 or 25
nucleomonomers in length.
In certain embodiments, the sense strand is a contiguous strand of nucleomonomers. In certain embodiments, the sense strand has one or more nicks.
In certain embodiments, the sense strand has one or more gaps. In certain embodiments, the one or more gaps, independently for each occurrence, comprise from 1 to 14 (or 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 1 1, 12, 13, or 14) unpaired nucleomonomers.
In certain embodiments, the nucleic acid has a blunt end.
In certain embodiments, the nucleic acid further comprises a 3 '-end overhang.
In anyj>ne of the embodiments described herein, the nucleic acid further comprises at least one hydroxymethyl substituted nucleomonomer. In a related embodiment, the at least one hydroxymethyl substituted nucleomonomer is selected from:
Monomer D r G
wherein, R is selected from a hydrogen, a methyl group, C(l , 2 ,3 ,4 ,5 ,6, 7, 8, 9, 10) alkyl, cholesterol, naturally or non-naturally occurring amino acid, sugar, vitamin, fluorophore, polyamine and fatty acid, and wherein the Base is nucleobase or analog thereof.
In certain embodiments, one or more of the at least one hydroxymethyl substituted nucleomonomers of the nucleic acid further comprise a 2'-0-methyl modification.
In certain embodiments, one or both of the last two positions at the 3'-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain embodiments, one or both of the last two positions at the 3 '-end of the antisense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain embodiments, any one or more of the last three positions at the 5 '-end of the sense strand is occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain
embodiments, at least one hydroxymethyl substituted nucleomonomer is in a double-stranded region of the nucleic acid.
In another embodiment, one or more nucleotides of the nucleic acid further comprises a 2'-modification of the sugar of the one or more nucleotides. In a related embodiment, the 2'- modification of the sugar of the one or more nucleotides is a 2'-0-methyl modification.
In another embodiment, the antisense strand comprises SEQ ID NOs: 5, 7, 13, 18, 20, 26, 31, 33, 39, 44, 46, 52, 57, 59, 65, 70, 72, 78, 83, 85, 91 , 96, 98, 104, 109, 1 1 1 , 1 17, 122, 124, 130, 135, 137, 143, 148, 150, 156, 161, 163, 169, 174, 176, 183, 188, 190, 196, 201, 203, 209, 214, 216, 222, 227, 229, 236, 241, 243, 249, 254, 256, 262, 267, 269, 275, 280, 282 or 288.
In another aspect, the instant disclosure provides for the use of a nucleic acid as disclosed herein for the manufacture of a medicament for use in the therapy of cancer.
In another aspect, the instant disclosure provides for a method for reducing the expression of a human HRAS gene, comprising administering a nucleic acid as disclosed herein to a cell expressing a HRAS gene, wherein the nucleic acid reduces expression of the HRAS gene in the cell. In a related embodiment, the cell is a human cell.
In another aspect, the instant disclosure provides for a method for treating or managing a disease or condition'in a subject associated, linked, and/or resulting from aberrant HRAS gene expression, comprising administering to the subject in need of treatment or management a nucleic acid comprising an antisense strand having a nucleic acid sequence selected from SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265 and 278, and a sense strand complementary to the antisense strand, wherein the antisense strand and the sense strand can anneal to from 15 base pairs to 60 base pairs, wherein the nucleic acid reduces the expression of the HRAS gene thereby treating or managing the disease or condition. In another aspect, the instant disclosure provide for a method for treating or managing a disease or condition in a subject associated, linked, and/or resulting from aberrant HRAS gene expression, comprising administering to the subject in need of treatment or management a nucleic acid as disclosed herein, wherein the nucleic acid reduces the expression of the HRAS gene thereby treating or managing the disease or condition.
In a related embodiment, the disease or condition is selected from one or more hyperproliferative diseases or disorders, leukemia, cutaneous melanoma, adenocarcinoma, squamous cell carcinoma, Philadelphia chromosome-negative myeloproliferative disorder, myelodysplasia syndrome, transitional cell carcinoma, ovarian cancer, brain tumors, breast cancer, bladder cancer, lung cancer, kidney tumors, urinary tract tumors, pancreatic carcinoma, and colorectal adenoma; as well as one or more angiogenic diseases or disorders, hepatocellular
carcinoma (HCC), NSCLC (lung nonsmall cell lung cancer), melanoma, colon cancer, prostate cancer, and glioblastoma;
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows examples of different architectures of hydroxymethyl substituted nucleomonomers that may be incorporated in the RNA complexes. Monomer A is shown for comparison and is a natural RNA monomer with its ribose scaffold. Monomers B-E contain a hydroxymethyl group substituent ("the free hydroxymethyl group"). The free hydroxymethyl group is for example attached at the C4' atom of a cyclic ribose scaffold or the C I ' atom of an acyclic ribose-based scaffold. The hydroxymethyl substituted nucleomonomers of the disclosure contain other oxygen atoms that are each attached to a phosphorus atom and thus partake in the formation of internucleotide linkages (see Figure 1). One or more of these other oxygen atoms can be part of a hydroxy group which is the case when one or more of the hydroxymethyl substituted nucleomonomers of the RNA complexes of the disclosure is (are) positioned at the 3'- or 5 '-end of an RNA strand. When one of the hydroxymethyl substituted nucleomonomers of the RNA complexes of the disclosure is positioned at the 3 '-end and/or the 5 '-end of the RNA strands, a hydroxyl group of this monomer can be phosphorylated, as can be the case for any terminally positioned natural RNA monomer. To the hydroxymethyl substituted
nucleomonomers of the disclosure is attached a nucleobase like uracil, thymine, cytosine, 5- methylcytosine, adenine, guanine or any other known natural or synthetic nucleobase or nucleobase analogue (designated as "Base" in Figure 1).
Figure 2 shows examples of derivatized, functionalized and conjugated variants of the hydroxymethyl substituted monomers. As examples are shown derivatized, functionalized and conjugated variants of the hydroxymethyl substituted 2', 3'-seco- monomer D (see Figure 1). Monomer F contains a group R linked via an ether linkage. Monomer G contains a group R linked via a thioether linkage. Monomer H contains a group R linked via an amide linkage. Monomer I contains a group R linked via an amino linkage. Monomer J contains a group R linked via a piperazino unit. By incorporation of one or several of such monomers into the RNA complexes of the disclosure, the properties of the RNA complexes can be modulated. For example, one or more such monomers may be introduced into the RNA complexes of the disclosure to increase biostability, increase RNA targeting capability, introduce specific delivery properties, and/or attach fluorescent groups for detection purposes.
Figure 3 shows structures of two of the hydroxymethyl substituted monomers (Monomer C and Monomer D) that may be a monomer of an oligonucleotide or RNA complex.
Figures 4a and 4b illustrate in vivo reduction of tumor growth in bladders of mice treated with HRAS RNA complexes. DETAILED DESCRIPTION
The present disclosure relates generally to compounds for use in treating disease by gene silencing and, more specifically, to a double-stranded nucleic acid complexes comprising an antisense strand and a continuous or a discontinuous passenger strand ("sense strand" containing a nick or gap) that decreases expression of a HRAS gene, and to uses of such nucleic acid complexes to treat, prevent or manage cancer associated with inappropriate HRAS gene expression. Further, the disclosure provides double-stranded nucleic acid complexes having one or more hydroxymethyl substituted nucleomonomer(s) and wherein one strand is complementary to a HRAS mRNA.
Definitions:
Prior to introducing more detail to this disclosure, it may be helpful to an appreciation thereof to provide definitions of certain terms to be used herein.
In the present description, any concentration range, percentage range, ratio range, or integer range is to be understood to include the value of any integer within the recited range and, when appropriate, fractions thereof (such as one tenth and one hundredth of an integer), unless otherwise indicated. Also, any number range recited herein relating to any physical feature, such as polymer subunits, size or thickness, are to be understood to include any integer within the recited range, unless otherwise indicated.
As used herein, "about" or "consisting essentially of mean ± 20% of the indicated range, value, or structure, unless otherwise indicated.
As used herein, the terms "include" and "comprise" are open ended and are used synonymously.
It should be understood that the terms "a" and "an" as used herein refer to "one or more" of the enumerated components.
The use of the alternative (e.g., "or") should be understood to mean either one, both, or any combination thereof of the alternatives.
As used herein, the term "linked" encompasses a covalent linkage either directly between two chemical entities (e.g., RNA and a hydroxymethyl substituted nucleomonomer), or indirectly between two chemical entities, for example via a linker.
As used herein, the term "overhang" (e.g., 3'-end overhang or 3' overhang) means an unpaired region of an RNA complex with may contain all nucleotides, non-nucleotides (e.g., hydroxymethyl substituted nucleomonomers), or a combination of nucleotides and non- nucleotides.
As used herein, the term "nucleobase analog" refers to a substituted or unsubstituted nitrogen-containing parent heteroaromatic ring that is capable of forming Watson-Crick hydrogen bonds with a complementary nucleobase or nucleobase analog. Exemplary nucleobase analogs include, but are not limited to, 7-deazaadenine, inosine, nebularine, nitropyrrole, nitroindole, 2-aminopurine, 2,6-diaminopurine, hypoxanthine, pseudouridine, 5- propynylcytidine, isocytidine, isoguanine, 7-deazaguanine, 2-thiopyrimidine, 6-thioguanine, 4- thiothymine, 4-thiouracil, 06-methyl guanine, N6-methyl adenine, 04-methyl thymine, 5,6- dihydrothymine, 5,6-dihydrouracil, 4-methylindole, ethenoadenine. Additional exemplary nucleobase analogs can be found in Fasman, 1989, Practical Handbook of Biochemistry and Molecular Biology, pp. 385-394, CRC Press, Boca Raton, Fla., and the references cited therein, incorporated herein by reference.
As used herein, the term "nucleomonomer" means a moiety comprising (1) a base covalently linked to (2) a second moiety. Nucleomonomers can be linked to form oligomers that bind to target or complementary base sequences in nucleic acids in a sequence specific manner. Nucleomonomers may be nucleosides, nucleotides, non-nucleotides or non-nucleosides (e.g. hydroxymethyl substituted nucleomonomer).
As used herein, the terms "hydroxymethyl substituted nucleomonomer", "hydroxymethyl nucleomonomer", "hydroxymethyl monomer", "acyclic nucleomonomer", "acyclic monomer", "acyclic hydroxymethyl substituted nucleomonomer" may be used interchangeably throughout.
As used herein, the terms "RISC length" or "RISC length RNA complex" means a nucleic acid molecule having less than 25 base pairs.
As used herein the terms "Dicer length" or "Dicer length RNA complex" means a nucleic acid molecule have 25 or more base pairs, generally, from 25 to 40 base pairs.
As used herein the term "bifunctional RNA complex" or "Afunctional dsRNA" means an RNA complex having a sense strand and antisense strand, wherein the sense strand and the antisense strand are each complementary to different regions of the same target RNA (i.e., a first
region and a second region), or are each complementary to a region of at least two different target RNAs.
As used herein, the term "isolated" means that the referenced material (e.g. , nucleic acid molecules of the instant disclosure), is removed from its original environment, such as being separated from some or all of the co-existing materials in a natural environment (e.g., a natural environment may be a cell).
As used herein, "complementary" refers to a nucleic acid molecule that can form hydrogen bond(s) with another nucleic acid molecule or itself by either traditional Watson-Crick base pairing or other non-traditional types of pairing (e.g., Hoogsteen or reversed Hoogsteen hydrogen bonding) between complementary nucleosides or nucleotides. In reference to the nucleic molecules of the present disclosure, the binding free energy for a nucleic acid molecule with its complementary sequence is sufficient to allow the relevant function of the nucleic acid molecule to proceed, for example, RNAi activity, and there is a sufficient degree of
complementarity to avoid non-specific binding of the nucleic acid molecule (e.g., dsRNA) to non-target sequences under conditions in which specific binding is desired, i.e., under physiological conditions in the case of in vivo assays or therapeutic treatment, or under conditions in which the assays are performed in the case of in vitro assays (e.g., hybridization assays). Determination of binding free energies for nucleic acid molecules is well known in the art (see, e.g., Turner et al., CSH Symp. Quant. Biol. LII: 123, 1987; Frier et al., Proc. Nat'l. Acad. Set. USA 83:9313, 1986; Turner et al., J. Am. Chem. Soc. 709:3783, 1987). Thus,
"complementary" or "specifically hybridizable" or "specifically binds" are terms that indicate a sufficient degree of complementarity or precise pairing such that stable and specific binding occurs between a nucleic acid molecule (e.g., dsRNA) and a DNA or RNA target. It is understood in the art that a nucleic acid molecule need not be 100% complementary to a target nucleic acid sequence to be specifically hybridizable or to specifically bind. That is, two or more nucleic acid molecules may be less than fully complementary and is indicated by a percentage of contiguous residues in a nucleic acid molecule that can form hydrogen bonds with a second nucleic acid molecule.
For example, a first nucleic acid molecule may have 10 nucleotides and a second nucleic acid molecule may have 10 nucleotides, then base pairing of 5, 6, 7, 8, 9, or 10 nucleotides between the first and second nucleic acid molecules, which may or may not form a contiguous double-stranded region, represents 50%, 60%, 70%, 80%, 90%, and 100% complementarity, respectively. In certain embodiments, complementary nucleic acid molecules may have wrongly paired bases - that is, bases that cannot form a traditional Watson-Crick base pair or other non-
traditional types of pair (i.e., "mismatched" bases). For instance, complementary nucleic acid molecules may be identified as having a certain number of "mismatches," such as zero or about 1, about 2, about 3, about 4 or about 5.
"Perfectly" or "fully" complementary nucleic acid molecules means those in which a certain number of nucleotides of a first nucleic acid molecule hydrogen bond (anneal) with the same number of residues in a second nucleic acid molecule to form a contiguous double-stranded region. For example, two or more fully complementary nucleic acid molecule strands can have the same number of nucleotides (i.e., have the same length and form one double-stranded region, with or without an overhang) or have a different number of nucleotides (e.g. , one strand may be shorter than but fully contained within another strand or one strand may overhang the other strand).
By "ribonucleic acid" or "RNA" is meant a nucleic acid molecule comprising at least one ribonucleotide molecule. As used herein, "ribonucleotide" refers to a nucleotide with a hydroxyl group at the 2'-position of a β-D-ribofuranose moiety. The term RNA includes double-stranded (ds) RNA, single-stranded (ss) RNA, isolated RNA (such as partially purified RNA, essentially pure RNA, synthetic RNA, recombinantly produced RNA), altered RNA (which differs from naturally occurring RNA by the addition, deletion, substitution or alteration of one or more nucleotides), or any combination thereof. For example, such altered RNA can include addition of non-nucleotide material, such as at one or both ends of an RNA molecule, internally at one or more nucleotides of the RNA, or any combination thereof. Nucleotides in RNA molecules of the instant disclosure can also comprise non-standard nucleotides, such as naturally occurring nucleotides, non-naturally occurring nucleotides, chemically-modified nucleotides,
deoxynucleotides, or any combination thereof. These altered RNAs may be referred to as analogs or analogs of RNA containing standard nucleotides (i.e., standard nucleotides, as used herein, are considered to be adenine, cytidine, guanidine, thymidine, and uridine).
The term "dsRNA" and "RNA complex" as used herein, refers to any nucleic acid molecule comprising at least one ribonucleotide molecule and capable of inhibiting or down regulating gene expression, for example, by promoting RNA interference ("RNAi") or gene silencing in a sequence-specific manner. The dsRNAs (mdRNAs) of the instant disclosure may be suitable substrates for Dicer or for association with RISC to mediate gene silencing by RNAi. Examples of dsRNA molecules of this disclosure are provided in the Sequence Listing identified herein. One or both strands of the dsRNA can further comprise a terminal phosphate group, such as a 5'-phosphate or 5', 3 '-diphosphate. As used herein, dsRNA molecules, in addition to at least one ribonucleotide, can further include substitutions, chemically-modified nucleotides, and non-
nucleotides. In certain embodiments, dsRNA molecules comprise ribonucleotides up to about 100% of the nucleotide positions.
The RNA complexes disclosed herein may comprise two strands that together constitute an siRNA duplex composed of an antisense strand (the antisense strand is also herein referred to as the guide strand) and a passenger strand (the passenger strand is also herein referred to as the sense strand), a single stranded RNA molecule (e.g. antisense RNA), a functional RNA (fRNA), or non-coding RNA (ncRNA), such as small temporal RNA (stRNA), microRNA (miRNA), small nuclear RNA (snRNA), short interfering RNA (siRNA), small nucleolar RNA (snRNA), ribosomal RNA (rRNA), transfer RNA (tRNA) and precursor RNAs thereof, an RNAa molecule, a microRNA mimicking molecule is also considered herein as an RNA complex of the disclosure, as is a single stranded antisense molecule that for example is useful for targeting microRNAs.
In addition, as used herein, the term dsRNA is meant to be equivalent to other terms used to describe nucleic acid molecules that are capable of mediating sequence specific RNAi, for example, meroduplex RNA (mdRNA), nicked dsRNA (ndsRNA), gapped dsRNA (gdsRNA), short interfering nucleic acid (siNA), siRNA, micro-RNA (miRNA), short hairpin RNA
(shRNA), short interfering oligonucleotide, short interfering substituted oligonucleotide, short interfering modified oligonucleotide, chemically-modified dsRNA, post-transcriptional gene silencing RNA (ptgsRNA), or the like. The term "large double-stranded RNA" ("large dsRNA") refers to any double-stranded RNA longer than about 40 base pairs (bp) to about 100 bp or more, particularly up to about 300 bp to about 500 bp. The sequence of a large dsRNA may represent a segment of an mRNA or an entire mRNA. A double-stranded structure may be formed by a self-complementary nucleic acid molecule or by annealing of two or more distinct
complementary nucleic acid molecule strands.
In addition, as used herein, the term "RNAi" is meant to be equivalent to other terms used to describe sequence specific RNA interference, such as post transcriptional gene silencing, translational inhibition, or epigenetics. For example, dsRNA molecules of this disclosure can be used to epigenetically silence genes at the post-transcriptional level or the pre-transcriptional level or any combination thereof.
As used herein, "target nucleic acid" refers to any nucleic acid sequence whose expression or activity is to be altered (e.g., HRAS). The target nucleic acid can be DNA, RNA, or analogs thereof, and includes single, double, and multi-stranded forms. By "target site" or "target sequence" is meant a sequence within a target nucleic acid (e.g., mRNA) that, when present in an RNA molecule, is "targeted" for cleavage by RNAi and mediated by a dsRNA
construct of this disclosure containing a sequence within the antisense strand that is
complementary to the target site or sequence.
As used herein, "off-target effect" or "off-target profile" refers to the observed altered expression pattern of one or more genes in a cell or other biological sample not targeted, directly or indirectly, for gene silencing by an mdRNA or dsRNA. For example, an off-target effect can be quantified by using a DNA microarray to determine how many non-target genes have an expression level altered by about two-fold or more in the presence of a candidate mdRNA or dsRNA, or analog thereof specific for a target sequence, such as a HRAS mRNA. A "minimal off-target effect" means that an mdRNA or dsRNA affects expression by about two-fold or more of about 25% to about 1 % of the non-target genes examined or it means that the off-target effect of substituted or modified mdRNA or dsRNA (e.g., having at least one uridine substituted with a 5-methyluridine or 2-thioribothymidine and optionally having at least one nucleotide modified at the 2'-position), is reduced by at least about 1 % to about 80% or more as compared to the effect on non-target genes of an unsubstituted or unmodified mdRNA or dsRNA.
By "sense region" or "sense strand" is meant one ore more nucleotide sequences of a dsRNA molecule having complementarity to one or more antisense regions of the dsRNA molecule. In addition, the sense region of a dsRNA molecule comprises a nucleic acid sequence having homology or identity to a target sequence, such as HRAS. By "antisense region" or "antisense strand" is meant a nucleotide sequence of a dsRNA molecule having complementarity to a target nucleic acid sequence, such as HRAS. In addition, the antisense region of a dsRNA molecule can comprise nucleic acid sequence region having complementarity to one or more sense strands of the dsRNA molecule.
"Analog" as used herein refers to a compound that is structurally similar to a parent compound (e.g., a nucleic acid molecule), but differs slightly in composition (e.g., one atom or functional group is different, added, or removed). The analog may or may not have different chemical or physical properties than the original compound and may or may not have improved biological or chemical activity. For example, the analog may be more hydrophilic or it may have altered activity as compared to a parent compound. The analog may mimic the chemical or biological activity of the parent compound (i.e., it may have similar or identical activity), or, in some cases, may have increased or decreased activity. The analog may be a naturally or non- naturally occurring (e.g., chemically-modified or recombinant) variant of the original compound. An example of an RNA analog is an RNA molecule having a non-standard nucleotide, such as 5-methyuridine or 5-methylcytidine or 2-thioribothymidine, which may impart certain desirable
properties (e.g., improve stability, bioavailability, minimize off-target effects or interferon response).
As used herein, the term "universal base" refers to nucleotide base analogs that form base pairs with each of the standard DNA/RNA bases with little discrimination between them. A universal base is thus interchangeable with all of the standard bases when substituted into a nucleotide duplex (see, e.g. , Loakes et al., J. Mol. Bio. 270:426, 1997). Exemplary universal bases include C-phenyl, C-naphthyl and other aromatic derivatives, inosine, azole carboxamides, or nitroazole derivatives such as 3-nitropyrrole, 4-nitroindole, 5-nitroindole, and 6-nitroindole (see, e.g., Loakes, Nucleic Acids Res. 29:2437, 2001).
The term "gene" as used herein, especially in the context of "target gene" or "gene target" for RNAi, means a nucleic acid molecule that encodes an RNA or a transcription product of such gene, including a messenger RNA (mRNA, also referred to as structural genes that encode for a polypeptide), an mRNA splice variant of such gene, a functional RNA (fRNA), or non-coding RNA (ncRNA), such as small temporal RNA (stRNA), microRNA (miRNA), small nuclear RNA (snRNA), short interfering RNA (siRNA), small nucleolar RNA (snRNA), ribosomal RNA (rRNA), transfer RNA (tRNA) and precursor RNAs thereof. Such non-coding RNAs can serve as target nucleic acid molecules for dsRNA mediated RNAi to alter the activity of the target RNA involved in functional or regulatory cellular processes.
As used herein, "gene silencing" refers to a partial or complete loss-of-function through targeted inhibition of gene expression in a cell, which may also be referred to as RNAi
"knockdown," "inhibition," "down-regulation," or "reduction" of expression of a target gene, such as a human HRAS gene. Depending on the circumstances and the biological problem to be addressed, it may be preferable to partially reduce gene expression. Alternatively, it might be desirable to reduce gene expression as much as possible. The extent of silencing may be determined by methods described herein and known in the art (see, e.g., PCT Publication No.
WO 99/32619; Elbashir et al, EMBO J. 20:6877, 2001). Depending on the assay, quantification of gene expression permits detection of various amounts of inhibition that may be desired in certain embodiments of this disclosure, including prophylactic and therapeutic methods, which will be capable of knocking down target gene expression, in terms of mRNA level or protein level or activity, for example, by equal to or greater than 10%, 30%, 50%, 75% 90%, 95% or 99% of baseline (i.e. , normal) or other control levels, including elevated expression levels as may be associated with particular disease states or other conditions targeted for therapy.
As used herein, the term "therapeutically effective amount" means an amount of dsRNA that is sufficient to result in a decrease in severity of disease symptoms, an increase in frequency
or duration of disease symptom-free periods, or a prevention of impairment or disability due to the disease, in the subject (e.g., human) to which it is administered. For example, a
therapeutically effective amount of dsRNA directed against an mRNA of HRAS (e.g., SEQ ID NO: 1) can inhibit the deposition of lipoproteins in the walls of arteries by at least about 20%, at least about 40%, at least about 60%, or at least about 80% relative to untreated subjects. A therapeutically effective amount of a therapeutic compound can decrease, for example, atheromatous plaque size or otherwise ameliorate symptoms in a subject. One of ordinary skill in the art would be able to determine such therapeutically effective amounts based on such factors as the subject's size, the severity of symptoms, and the particular composition or route of administration selected. The nucleic acid molecules of the instant disclosure, individually, or in combination or in conjunction with other drugs, can be used to treat diseases or conditions discussed herein. For example, to treat a particular disease, disorder, or condition, the dsRNA molecules can be administered to a patient or can be administered to other appropriate cells evident to those skilled in the art, individually or in combination with one or more drugs, under conditions suitable for treatment.
In addition, it should be understood that the individual compounds, or groups of compounds, derived from the various combinations of the structures and substituents described herein, are disclosed by the present application to the same extent as if each compound or group of compounds was set forth individually. Thus, selection of particular structures or particular substituents is within the scope of the present disclosure. As described herein, all value ranges are inclusive over the indicated range. Thus, a range of C 1 -C4 will be understood to include the values of 1, 2, 3, and 4, such that Ci, C2, C3 and C4 are included.
The term "alkyl" as used herein refers to a saturated, branched or unbranched, substituted or unsubstituted aliphatic group containing from 1-22 carbon atoms (1, 2, 3, 4, 5 ,6, 7, 8, 9, 10, 1 1, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, or 22 carbon atoms). This definition applies to the alkyl portion of other groups such as, for example, alkoxy, alkanoyl, aralkyl, and other groups defined below. The term "cycloalkyl" as used herein refers to a saturated, substituted or unsubstituted cyclic alkyl ring containing from 3 to 12 carbon atoms.
The term "alkenyl" as used herein refers to an unsaturated, branched or unbranched, substituted or unsubstituted alkyl or cycloalkyl having 2 to 22 carbon atoms and at least one carbon-carbon double bond. The term "alkynyl" as used herein refers to an unsaturated, branched or unbranched, substituted or unsubstituted alkyl or cycloalkyl having 2 to 22 carbon atoms and at least one carbon-carbon triple bond.
The term "alkoxy" as used herein refers to an alkyl, cycloalkyl, alkenyl, or alkynyl group covalently bonded to an oxygen atom. The term "alkanoyl" as used herein refers to -C(=0)-alkyl, which may alternatively be referred to as "acyl." The term "alkanoyloxy" as used herein refers to - 0-C(=0)-alkyl groups. The term "alkylamino" as used herein refers to the group -NRR', where R and R' are each either hydrogen or alkyl, and at least one of R and R' is alkyl. Alkylamino includes groups such as piperidino wherein R and R' form a ring. The term "alkylaminoalkyl" refers to -alkyl-NRR'.
The term "aryl" as used herein refers to any stable monocyclic, bicyclic, or polycyclic carbon ring system of from 4 to 12 atoms in each ring, wherein at least one ring is aromatic. Some examples of an aryl include phenyl, naphthyl, tetrahydro-naphthyl, indanyl, and biphenyl. Where an aryl substituent is bicyclic and one ring is non-aromatic, it is understood that attachment is to the aromatic ring. An aryl may be substituted or unsubstituted.
The term "heteroaryl" as used herein refers to any stable monocyclic, bicyclic, or polycyclic carbon ring system of from 4 to 12 atoms in each ring, wherein at least one ring is aromatic and contains from 1 to 4 heteroatoms selected from oxygen, nitrogen and sulfur. Some examples of a heteroaryl include acridinyl, quinoxalinyl, pyrazolyl, indolyl, benzotriazolyl, furanyl, thienyl, benzothienyl, benzofuranyl, quinolinyl, isoquinolinyl, oxazolyl, isoxazolyl, pyrazinyl, pyridazinyl, pyridinyl, pyrimidinyl, pyrrolyl, and tetrahydroquinolinyl. A heteroaryl includes the N-oxide derivative of a nitrogen-containing heteroaryl.
The term "heterocycle" or "heterocyclyl" as used herein refers to an aromatic or nonaromatic ring system of from five to twenty-two atoms, wherein from 1 to 4 of the ring atoms are heteroatoms selected from oxygen, nitrogen, and sulfur. Thus, a heterocycle may be a heteroaryl or a dihydro or tetrathydro version thereof.
The term "aroyl" as used herein refers to an aryl radical derived from an aromatic carboxylic acid, such as a substituted benzoic acid. The term "aralkyl" as used herein refers to an aryl group bonded to an alkyl group, for example, a benzyl group.
The term "carboxyl" as used herein represents a group of the formula -C(=0)OH or -C(=0)0". The terms "carbonyl" and "acyl" as used herein refer to a group in which an oxygen atom is double-bonded to a carbon atom >C=0. The term "hydroxyl" as used herein refers to -OH or -O". The term "nitrile" or "cyano" as used herein refers to -CN. The term "halogen" or "halo" refers to fluoro (-F), chloro (-C1), bromo (-Br), and iodo (-Γ).
The term "cycloalkyl" as used herein refers to a saturated cyclic hydrocarbon ring system containing from 3 to 12 carbon atoms that may be optionally substituted. Exemplary embodiments include, but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. In certain
embodiments, the cycloalkyl group is cyclopropyl. In another embodiment, the (cycloalkyl)alkyl groups contain from 3 to 12 carbon atoms in the cyclic portion and 1 to 6 carbon atoms in the alkyl portion. In certain embodiments, the (cycloalkyl)alkyl group is cyclopropylmethyl. The alkyl groups are optionally substituted with from one to three substituents selected from the group consisting of halogen, hydroxy and amino.
The terms "alkanoyl" and "alkanoyloxy" as used herein refer, respectively, to -C(0)-alkyl groups and -0-C(=0)- alkyl groups, each optionally containing 2 to 10 carbon atoms. Specific embodiments of alkanoyl and alkanoyloxy groups are acetyl and acetoxy, respectively.
The term "alkynyl" as used herein refers to an unsaturated branched, straight-chain, or cyclic alkyl group having 2 to 10 carbon atoms and having at least one carbon-carbon triple bond derived by the removal of one hydrogen atom from a single carbon atom of a parent alkyne. Exemplary alkynyls include ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl, 1- pentynyl, 2-pentynyl, 4-pentynyl, 1-octynyl, 6-methyl-1-heptynyl, 2-decynyl, or the like. The alkynyl group may be substituted or unsubstituted.
The term "hydroxyalkyl" alone or in combination, refers to an alkyl group as previously defined, wherein one or several hydrogen atoms, preferably one hydrogen atom has been replaced by a hydroxyl group. Examples include hydroxymethyl, hydroxyethyl and 2- hydroxyethyl.
The term "aminoalkyl" as used herein refers to the group -NRR', where R and R' may independently be hydrogen or (C1-C4) alkyl.
The term "alkylaminoalkyl" refers to an alkylamino group linked via an alkyl group (i.e., a group having the general structure -alkyl-NH-alkyl or -alkyl-N(alkyl)(alkyl)). Such groups include, but are not limited to, mono- and di-(Ci-Cg alky aminoCi-Cg alkyl, in which each alkyl may be the same or different.
The term "dialkylaminoalkyl" refers to alkylamino groups attached to an alkyl group.
Examples include, but are not limited to, Ν,Ν-dimethylaminomethyl, N,N-dimethylaminoethyl Ν,Ν-dimethylaminopropyl, and the like. The term dialkylaminoalkyl also includes groups where the bridging alkyl moiety is optionally substituted.
The term "haloalkyl" refers to an alkyl group substituted with one or more halo groups, for example chloromethyl, 2-bromoethyl, 3-iodopropyl, trifluoromethyl, perfluoropropyl, 8- chlorononyl, or the like.
The term "carboxyalkyl" as used herein refers to the substituent -R10-COOH, wherein R10 is alkylene; and "carbalkoxyalkyl" refers to -R10-C(=O)ORu, wherein R10 and R1 1 are alkylene and alkyl respectively. In certain embodiments, alkyl refers to a saturated straight- or branched-
chain hydrocarbyl radical of 1 to 6 carbon atoms such as methyl, ethyl, n-propyl, isopropyl, n- butyl, t-butyl, n-pentyl, 2-methylpentyl, n-hexyl, and so forth. Alkylene is the same as alkyl except that the group is divalent.
The term "alkoxy" includes substituted and unsubstituted alkyl, alkenyl, and alkynyl groups covalently linked to an oxygen atom. In one embodiment, the alkoxy group contains 1 to about 10 carbon atoms. Embodiments of alkoxy groups include, but are not limited to, methoxy, ethoxy, isopropyloxy, propoxy, butoxy, and pentoxy groups. Embodiments of substituted alkoxy groups include halogenated alkoxy groups. In a further embodiment, the alkoxy groups can be substituted with groups such as alkenyl, alkynyl, halogen, hydroxyl, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy, aryloxycarbonyloxy, carboxylate, alkylcarbonyl, arylcarbonyl, alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl, alkylthiocarbonyl, alkoxyl, phosphate, phosphonato, phosphinato, cyano, amino (including alkylamino, dialkylamino, arylamino, diarylamino, and alkylarylamino), acylamino (including alkylcarbonylamino, arylcarbonylamino, carbamoyl and ureido), amidino, imino, sulfhydryl, alkylthio, arylthio, thiocarboxylate, sulfates, alkylsulfinyl, sulfonato, sulfamoyl, sulfonamide, nitro, trifluoromethyl, cyano, azido, heterocyclyl, alkylaryl, or an aromatic or heteroaromatic moieties. Exemplary halogen substituted alkoxy groups include, but are not limited to, fluoromethoxy, difluoromethoxy, trifluoromethoxy, chloromethoxy, dichloromethoxy, and trichloromethoxy.
The term "alkoxyalkyl" refers to an alkylene group substituted with an alkoxy group. For example, methoxyethyl (CH3OCH2CH2-) and ethoxymethyl (CH3CH2OCH2-) are both C3 alkoxyalkyl groups.
The term "aroyl, " as used alone or in combination herein, refers to an aryl radical derived from an aromatic carboxylic acid, such as optionally substituted benzoic or naphthoic acids.
The term "aralkyl" as used herein refers to an aryl group bonded to the 2-pyridinyl ring or the 4-pyridinyl ring through an alkyl group, preferably one containing 1 to 10 carbon atoms. A preferred aralkyl group is benzyl.
The term "carboxy," as used herein, represents a group of the formula -C(=0)OH or - C(=0)CT.
The term "carbonyl" as used herein refers to a group in which an oxygen atom is double-bonded to a carbon atom -C=0.
The term "trifluoromethyl" as used herein refers to -CF3.
The term "trifluoromethoxy" as used herein refers to -OCF3.
The term "hydroxyl" as used herein refers to -OH or -O".
The term "nitrile" or "cyano" as used herein refers to the group -CN.
The term "nitro," as used herein alone or in combination refers to a -N02 group.
The term "amino" as used herein refers to the group -NR R9, wherein R9 may independently be hydrogen, alkyl, aryl, alkoxy, or heteroaryl. The term "aminoalkyl" as used herein represents a more detailed selection as compared to "amino" and refers to the
group -NR'R', wherein R' may independently be hydrogen or (C1-C4) alkyl. The term
"dialkylamino" refers to an amino group having two attached alkyl groups that can be the same or different.
The term "alkanoylamino" refers to alkyl, alkenyl or alkynyl groups containing the group -C(=0)- followed by -N(H)-, for example acetylamino, propanoylamino and butanoylamino and the like.
The term "carbonylamino" refers to the group -NR'-CO-CH2-R', wherein R' may be independently selected from hydrogen or (C1-C4) alkyl.
The term "carbamoyl" as used herein refers to -0-C(0)NH2.
The term "carbamyl" as used herein refers to a functional group in which a nitrogen atom is directly bonded to a carbonyl, i.e., as in -NR"C(=0)R" or -C(=0)NR"R", wherein R" can be independently hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkoxy, cycloalkyl, aryl, heterocyclo, or heteroaryl.
The term "alkylsulfonylamino" refers to the group -NHS(0)2R12, wherein R12 is alkyl. The term "halogen" as used herein refers to bromine, chlorine, fluorine or iodine. In one embodiment, the halogen is fluorine. In another embodiment, the halogen is chlorine.
The term "heterocyclo" refers to an optionally substituted, unsaturated, partially saturated, or fully saturated, aromatic or nonaromatic cyclic group that is a 4 to 7 membered monocyclic, or 7 to 11 membered bicyclic ring system that has at least one heteroatom in at least one carbon atom- containing ring. The substituents on the heterocyclo rings may be selected from those given above for the aryl groups. Each ring of the heterocyclo group containing a heteroatom may have 1, 2, or 3 heteroatoms selected from nitrogen, oxygen or sulfur. Plural heteroatoms in a given heterocyclo ring may be the same or different.
Exemplary monocyclic heterocyclo groups include pyrrolidinyl, pyrrolyl, indolyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, thiazolyl, furyl, tetrahydrofuryl, thienyl, piperidinyl, piperazinyl, azepinyl, pyrimidinyl, pyridazinyl, tetrahydropyranyl, morpholinyl, dioxanyl, triazinyl and triazolyl. Preferred bicyclic heterocyclo groups include benzothiazolyl, benzoxazolyl, benzothienyl, quinolinyl, tetrahydroisoquinolinyl, benzimidazolyl, benzofuryl, indazolyl,
benzisothiazolyl, isoindolinyl and tetrahydroquinolinyl. In more detailed embodiments heterocyclo groups may include indolyl, imidazolyl, furyl, thienyl, thiazolyl, pyrrolidyl, pyridyl and pyrimidyl.
The "percent identity" between two or more nucleic acid sequences is a function of the number of identical positions shared by the sequences (i.e., % identity = number of identical positions / total number of positions x 100), taking into account the number of gaps, and the length of each gap that needs to be introduced to optimize alignment of two or more sequences. The comparison of sequences and determination of percent identity between two or more sequences can be accomplished using a mathematical algorithm, such as BLAST and Gapped BLAST programs at their default parameters (e.g., BLASTN, see Altschul et al., J. Mol. Biol. 275:403-410, 1990).
"Aptamer" or "nucleic acid aptamer" as used herein is meant a nucleic acid molecule that binds specifically to a target molecule wherein the nucleic acid molecule has sequence that comprises a sequence recognized by the target molecule in its natural setting. Alternately, an aptamer can be a nucleic acid molecule that binds to a target molecule wherein the target molecule does not naturally bind to a nucleic acid. The target molecule can be any molecule of interest. For example, the aptamer can be used to bind to a ligand-binding domain of a protein, thereby preventing interaction of the naturally occurring ligand with the protein. This is a non- limiting example and those in the art will recognize that other embodiments can be readily generated using techniques generally known in the art (see, e.g., Gold et al., Annu. Rev.
Biochem. 64:163, 1995; Brody and Gold, J. Biotechnol. 74:5, 2000; Sun, Curr. Opin. Mol. Ther. 2: 100, 2000; Kusser, J. Biotechnol. 74:21, 2000; Hermann and Patel, Science 257:820, 2000; and Jayasena, Clinical Chem. 45: 1628, 1999).
The term "substituted" as used herein refers to an atom having one or more substitutions or substituents which can be the same or different and may include a hydrogen substituent. Thus, the terms alkyl, cycloalkyl, alkenyl, alkynyl, alkoxy, alkanoyl, alkanoyloxy, alkylamino, alkylaminoalkyl, aryl, heteroaryl, heterocycle, aroyl, and aralkyl as used herein refer to groups which include substituted variations. Substituted variations include linear, branched, and cyclic variations, and groups having a substituent or substituents replacing one or more hydrogens attached to any carbon atom of the group. Substituents that may be attached to a carbon atom of the group include alkyl, cycloalkyl, alkenyl, alkynyl, alkoxy, alkanoyl, alkanoyloxy, alkylamino, alkylaminoalkyl, aryl, heteroaryl, heterocycle, aroyl, aralkyl, acyl, hydroxyl, cyano, halo, haloalkyl, amino, aminoacyl, alkylaminoacyl, acyloxy, aryloxy, aryloxyalkyl, mercapto, nitro, carbamyl, carbamoyl, and heterocycle. For example, the term ethyl includes without limitation -CH2CH3, -CHFCH3, -CF2CH3, -CHFCH2F, -CHFCHF2, -CHFCF3, -CF2CH2F, -CF2CHF2,
-CF2CF3, and other variations as described above. Representative substituents include -X, -R6, -0-, =0, -OR, -SR6, -S-, =S, -NR6R6, =NR6, -CX3, -CF3, -CN, -OCN, -SCN, -NO, -N02) =N2, -N3, -S(=0)20-, -S(=0) 2OH, -S(=0)2R6, -OS(=0)20-, -OS(=0)2OH, -OS(=0)2R6, -P(=0)(0 )2, -P(=0)(OH)(0~), -0P(=0)2(0-), -C(-0)R6, -C(=S)R6, -C(=0)OR6, -C(=0)0-, -C(=S)OR6, -NR6-C(=0)-N(R6)2, -NR6-C(=S)-N(R6)2, and -C(=NR6)NR6R6, wherein each X is independently a halogen; and each R6 is independently hydrogen, halogen, alkyl, aryl, arylalkyl, arylaryl, arylheteroalkyl, heteroaryl, heteroarylalkyl, NR7R7, -C(=0)R7, and -S(=0)2R7; and each R7 is independently hydrogen, alkyl, alkanyl, alkynyl, aryl, arylalkyl, arylheteralkyl, arylaryl, heteroaryl or heteroarylalkyl. Aryl containing substituents, whether or not having one or more substitutions, may be attached in a para (/?-), meta (m-) or ortho (o-) conformation, or any combination thereof. In general, substituents may be further substituted with any atom or group of atoms.
As used herein, the term "homo," when referring to an amino acid, means that an additional carbon is added to the side chain, while the term "nor," when referring to an amino acid, means that a carbon is subtracted from the side chain. Thus, homolysine refers to side chain-(CH2)5NH2.
The term "carrier" as used herein refers to any non-nucleic acid compound in a composition or formulation.
The term "constitutively cationic" as used herein refers to a compound acting as a base with a pKa greater than 9.
The term "constitutively anionc" as used herein refers to a compound acting as an acid with a pKa less than 4.
The term "constitutively neutral" as used herein refers to a zwitterionic compound or a compound with no acid or base functionality.
The term "nanoparticle forming compound" or "nanoparticle forming agent" as used herein refers to a compound that is capable of forming a nanoparticle either alone or when combined with another compound described herein. By way of example only, nanoparticle forming compound include, but are not limited to, lipids, cationic lipids, non-cationic lipids, anionic lipids, neutral lipids, zwitterionic lipids, compounds with lipophilic proproperties, peptides, proteins, polymers, and DILA2 amino acid compounds. The term "nanoparticle" and "particle" may be used interchangeably throughout this disclosure.
The term "N P ratio" as used herein refers to the ratio of the total moles of nitrogen to the total moles of phosphates of a nucleic acid (e.g., siRNA) in a composition or formulation.
The term "charge ratio" as used herein refers to the ratio of the total moles of cations to the total moles of anions in a composition or formulation at a specified pH.
The term "C N ratio" or "charged carrier to nucleic acid ratio" as used herein refers to the (total moles of carrier cations minus the total moles of carrier anions) divided by the total moles of phosphates of a nucleic acid (e.g., siRNA) in a composition or formulation.
The term "delivery efficiency ratio" or "DER" refers to the ratio of the total mass of the carrier compounds in the composition or formulation to the total mass of nucleic acids in the composition or formulation.
The term "carrier charge ratio" refers to the ratio of the total moles of cationic carrier to total moles of anionic carrier.
The term "delta charge ratio" or Δ charge ratio" refers to the charge ratio of the composition or formulation at pH 4 minus the charge ratio of the same composition or formulation at pH 7.
RAS viral (v-ras) oncogene homolog (RAS)
The product of the v-Ha-ras Harvey rat sarcoma viral oncogene homolog gene (HRAS; also known as RASHl , c-bas/has, HRAS-1, and HRAS-2) is a GTPase protein that responds to a large number of signals and plays a central role in transducing signals that regulate cell proliferation, survival, differentiation, development, growth, fertility, and apoptosis. Mutation or overexpression of HRAS that increases activity is associated with a variety of disorders including one or more hyperproliferative diseases or disorders, for example, leukemia, cutaneous melanoma, adenocarcinoma, squamous cell carcinoma, Philadelphia chromosome-negative myeloproliferative disorder, myelodysplastic syndrome, transitional cell carcinoma, ovarian cancer, brain tumors, breast cancer, bladder cancer, lung cancer, kidney tumors, urinary tract tumors, pancreatic carcinoma, and colorectal adenoma; as well as one or more angiogenic diseases or disorders.
More detail regarding HRAS and related diseases or disorders is described at in the Online Mendelian Inheritance in Man database (OMIM Accession No. 190020). The complete mRNA sequence for human HRAS has Genbank accession number NM_005343.2 (SEQ ID NO: 1). As used herein, reference to RAS mRNA or RNA sequences or sense strands means an HRAS as set forth in SEQ ED NO: 1 , as well as isoforms, variants, and homologs having at least 80% or more identity with human HRAS sequence as set forth in SEQ ID NO: 1.
UGCCCUGCGCCCGCAACCCGAGCCGCACCCGCCGCGGACGGAGCCCAUGCGCGGG GCGAACCGCGCGCCCCCGCCCCCGCCCCGCCCCGGCCUCGGCCCCGGCCCUGGCCC
CGGGGGCAGUCGCGCCUGUGAACGGUGGGGCAGGAGACCCUGUAGGAGGACCCCG GGCCGCAGGCCCCUGAGGAGCGAUGACGGAAUAUAAGCUGGUGGUGGUGGGCGC CGGCGGUGUGGGCAAGAGUGCGCUGACCAUCCAGCUGAUCCAGAACCAUUUUGU GGACGAAUACGACCCCACUAUAGAGGAUUCCUACCGGAAGCAGGUGGUCAUUGA UGGGGAGACGUGCCUGUUGGACAUCCUGGAUACCGCCGGCCAGGAGGAGUACAG CGCCAUGCGGGACCAGUACAUGCGCACCGGGGAGGGCUUCCUGUGUGUGUUUGCC AUCAACAACACCAAGUCUUUUGAGGACAUCCACCAGUACAGGGAGCAGAUCAAAC GGGUGAAGGACUCGGAUGACGUGCCCAUGGUGCUGGUGGGGAACAAGUGUGACC UGGCUGCACGCACUGUGGAAUCUCGGCAGGCUCAGGACCUCGCCCGAAGCUACGG C AUCCCCUAC AUCGAGACCUCGGCC AAGACCCGGC AGGGAGUGG AGGAUGCCUUC UACACGUUGGUGCGUGAGAUCCGGCAGCACAAGCUGCGGAAGCUGAACCCUCCUG AUGAGAGUGGCCCCGGCUGCAUGAGCUGCAAGUGUGUGCUCUCCUGACGCAGCAC AAGCUCAGGACAUGGAGGUGCCGGAUGCAGGAAGGAGGUGCAGACGGAAGGAGG AGGAAGGAAGGACGGAAGCAAGGAAGGAAGGAAGGGCUGCUGGAGCCCAGUCAC CCCGGGACCGUGGGCCGAGGUG ACUGC AGACCCUCCC AGGGAGGCUGUGC ACAGA CUGUCUUGAACAUCCCAAAUGCCACCGGAACCCCAGCCCUUAGCUCCCCUCCCAG GCCUCUGUGGGCCCUUGUCGGGCACAGAUGGGAUCACAGUAAAUUAUUGGAUGG UCUUGAAAAAAAAAAAAAAAAAA (SEQ ID NO: 1) Nucleic Acid Compounds For Regulating HRAS mRNA Expression and Uses Thereof
In one aspect, the instant disclosure provides a nucleic acid that down regulates the expression of a v-Ha-ras Harvey rat sarcoma viral oncogene homolog gene (HRAS) mRNA, the nucleic acid comprising an antisense strand having a nucleic acid sequence selected from SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278, and a sense strand complementary to the antisense strand, wherein the antisense strand and the sense strand can anneal to form a double-stranded region of 15 base pairs to 60 base pairs.
In one aspect, the instant disclosure provides a nucleic acid that down regulates the expression of a v-Ha-ras Harvey rat sarcoma viral oncogene homolog gene (HRAS) mRNA, the nucleic acid comprising an antisense strand having a region of 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24 ,25 ,26, 27, 28, 29, 30, 31 , 32, 33, 34, 35 ,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 , 52, 53, 54, 55, 56, 57, 58, 59, or 60 contiguous nucleomonomers, wherein at least 15 contiguous nucleomonomers of the nucleic acid correspond to 15 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81 , 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225,
239, 252, 265, and 278, and a sense strand complementary to the antisense strand, wherein the antisense strand and the sense strand can anneal to form 15 base pairs to 60 base pairs. In a related embodiment, the nucleic acid has at least 16 contiguous nucleomonomers of the nucleic acid correspond to 16 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278. In a related embodiment, the nucleic acid has at least 17 contiguous nucleomonomers of the nucleic acid correspond to 17 contiguous nucleomonomers of SEQ ED NOs:. In a related embodiment, the nucleic acid has at least 18 contiguous nucleomonomers of the nucleic acid correspond to 18 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81 , 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278. In a related embodiment, the nucleic acid has at least 19 contiguous nucleomonomers of the nucleic acid correspond to 19 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278. In a related embodiment, the nucleic acid has at least 20 contiguous nucleomonomers of the nucleic acid correspond to 20 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278. In a related embodiment, the nucleic acid has at least 21 contiguous nucleomonomers of the nucleic acid correspond to 21 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278. In a related embodiment, the nucleic acid has at least 22 contiguous nucleomonomers of the nucleic acid correspond to 22 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278.
In certain embodiments, the nucleic acid is a ribonucleic acid. In certain embodiments, the ribonucleic acid is a siRNA.
In certain embodiments, the antisense strand is 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24 ,25
,26, 27, 28, 29, 30, 31 , 32, 33, 34 ,35 ,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, or 60 nucleomonomers in length. In other embodiments, the antisense strand is 18, 19, 20, 21, 22, 23, 24 or 25 nucleomonomers in length. In certain embodiments, the antisense strand is 19, 20, or 21 nucleomonomers in length
In certain embodiments, the sense strand is a contiguous strand of nucleomonomers.
In certain embodiments, the sense strand has one or more nicks.
In certain embodiments, the sense strand has one or more gaps. In certain embodiments, the one or more gaps, independently for each occurrence, comprise from 1 , 2, 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, or 14 unpaired nucleomonomers.
In certain embodiments, the nucleic acid has a blunt end.
In certain embodiments, the nucleic acid further comprises a 3'-end overhang.
In any one of the embodiments described herein, the nucleic acid further comprises at least one hydroxymethyl substituted nucleomonomer. In a related embodiment, the hydroxymethyl substituted nucleomonomer is selected from:
wherein, R is selected from a hydrogen, a methyl group, C(l , 2 ,3 ,4 ,5 ,6, 7, 8, 9, 10) alkyl, cholesterol, naturally or non-naturally occurring amino acid, sugar, vitamin, fluorophore, polyamine and fatty acid, and wherein the Base is nucleobase or analog thereof.
In certain embodiments, one or both of the last two positions at the 3 '-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain embodiments, one or both of the last two positions at the 3'-end of the antisense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain embodiments, any one or more of the last three positions at the 5 '-end of the sense strand is occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain embodiments, at least one hydroxymethyl substituted nucleomonomer is in a double-stranded region of the nucleic acid.
In one aspect, the disclosure provide for a nucleic acid comprising a sense strand and an antisense strand, and a double-stranded region having from 15 to 24 base pairs, wherein any one
or more of the last three positions at the 5 '-end of the sense strand is occupied by the same or different hydroxymethyl substituted nucleomonomer.
In another aspect, the nucleic acid further comprises that one or both of the last two positions of the 3 '-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer.
In yet another aspect, the nucleic acid further comprises that one or both of the last two positions of the 3 '-end of the antisense strand is occupied by the same or different
hydroxymethyl substituted nucleomonomer.
In another aspect, the disclosure provide for a nucleic acid comprising a sense strand and an antisense strand, and a double-stranded region having from 15 to 24 base pairs, wherein one or more of positions 5, 6, 7 and 8 of the antisense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer, wherein the positions of the antisense strand are numbered beginning with position 1 at the 5'end of the antisense strand.
In another aspect, the nucleic acid further comprises that one or both of the last two positions of the 3 '-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer.
In yet another aspect, the nucleic acid further comprises that one or both of the last two positions of the 3 '-end of the antisense strand is occupied by the same or different
hydroxymethyl substituted nucleomonomer.
In another aspect, the nucleic acid has a double-stranded region of 19 or 20 base pairs.
In another aspect, the sense strand and the antisense strand are each 21 or 22
nucleomonomers in length.
In another aspect, the nucleic acid has a blunt end or a 3 '-end overhang.
In another aspect, the antisense strand has a region of at least 15 contiguous
nucleomonomers corresponding to any 15 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278.
In a related aspect, the antisense strand has a region of at least 15, 16, 17, 18, 19, 20, 21,
22, 23, or 24 contiguous nucleomonomers corresponding to any 15, 16, 17, 18, 19, 20, 21, 22,
23, or 24 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278.
In one aspect, this disclosure provides for a nucleic acid comprising a sense strand and an antisense strand, and a double-stranded region having from 25 to 40 base pairs, wherein the last position of the 3 '-end of the antisense strand and the last position of the 3 '-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer.
In another aspect, the last two positions of the 3 '-end of the antisense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer.
In one aspect, this disclosure provide for a nucleic acid comprising a sense strand and an antisense strand, and a double-stranded region having from 25 to 40 base pairs, wherein one or more of positions 21 , 22 and 23 of the sense strand is occupied by the same or different hydroxymethyl substituted nucleomonomer, wherein the positions of the sense strand are numbered beginning with position 1 at the 5 '-end of the sense strand.
In one aspect, this disclosure provide for a nucleic acid comprising a sense strand and an antisense strand, and a double-stranded region having from 25 to 40 base pairs, wherein one or more of positions 18, 19, 20, 21 , and 22 of the antisense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer, wherein the positions of the sense strand are numbered beginning with position 1 at the 3 '-end of the antisense strand.
In another aspect, the nucleic acid further comprises that one or both of the last two positions of the 3 '-end of the antisense strand are occupied by the same or different
hydroxymethyl substituted nucleomonomer.
In another aspect, the nucleic acid further comprises that one or both of the last two positions of the 3 '-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer.
In another aspect, the antisense strand has a region of at least 15 contiguous
nucleomonomers corresponding to any 15 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81 , 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278.
In a related aspect, the antisense strand has a region of at least 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 or 40 contiguous
nucleomonomers corresponding to any 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 or 40 contiguous nucleomonomers of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81 , 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278.
In another aspect, the hydroxymethyl substituted nucleomonomer is a 2'-3'-seco- nucleomonomer.
In another aspect, the hydroxymethyl substituted nucleomonomer is selected from monomers D, F, G, H, I, or J:
wherein R is selected from the group consisting of a hydrogen, an alkyl group, a cholesterol derivative, a fluorophore, a polyamine, a fatty acid, an amino acid, a saccharide, and a polypeptide, wherein Base is any purine, pyrimidine, or derivative or analogue thereof.
In another aspect, the nucleic acid further comprises a nucleotide analogue selected from the group consisting of 2'-0-alkyl-RNA monomers, 2'-amino-DNA monomers, 2'-fluoro-DNA monomers, LNA monomers, PNA monomers, HNA monomers, ANA monomers, FANA monomers, CeNA monomers, ENA monomers, DNA monomers, and INA monomers.
In another aspect, the instant disclosure provides for the use of a nucleic acid as disclosed herein for the manufacture of a medicament for use in the therapy of cancer.
For example purposes only, the positions of the sense strand may be described as follows where X represents a nucleomonomer (nucleoside or hydroxymethyl substituted
nucleomonomer) and the number represents the position of that nucleomonomer in the strand. For a RISC length RNA complex, n may be from 5 to 14 (or 5, 6, 7, 8, 9, 10, 1 1, 12, 13 or 14), and for a Dicer length RNA complex, n may be from 15 to 30 (or 15, 16, 17, 18, 19, 20, 21 , 22, 23, 24, 25, 26, 27, 28, 29 or 30). The same procedure for determining the position of a nucleomonomer in sense strand may be applied to the antisense strand.
5' Xl -X2-X3-X4-X5-X6-X7-X8-X9-X10-Xn 3'
In this example, nucleomonomer XI occupies position 1, X2 occupies position 2.
In a related aspect, the last two nucleomonomers of the 3'-end of the antisense strand and the last two nucleomonomers of the 3 '-end of the sense strand are hydroxymethyl substituted nucleomonomers.
For example purposes only, the position of the hydroxymethyl substituted
nucleomonomers in each of the sense strand and the antisense strand may be represented as follows where X represents a nucleomonomer (nucleoside or hydroxymethyl substituted nucleomonomer) and n represents the position. For a RISC length RNA complex, n may be froml3 to 22 (or 13, 14, 15, 16, 17, 18, 19, 20, 21 or 22), and for a Dicer length RNA complex, n may be from 23 to 38 (or 23, 24, 25, 26, 27, 28, 29, 30, 31 , 32, 33, 34, 36, 37 or 38).
In this example, the last nucleomonomer is represented by position X(n+2), the next to last nucleomonomer is represented by position X(n+i), and the last two nucleomonomers of the 3'-end of the strand (whether the sense strand or the antisense strand) are represented by X(n+i) and
In a related aspect, one or more hydroxymethyl substituted nucleomonomer(s) are at one or more of positions 5, 6, 7 or 8 counting from the 5'-end of the antisense strand.
In a related aspect, one or more hydroxymethyl substituted nucleomonomer(s) are at position 7 counting from the 5 '-end of the antisense strand.
In a related aspect, the double-stranded region has 19 or 20 base pairs.
In a related aspect, the sense strand and the antisense strand each have 21 or 22 nucleomonomers.
In a related aspect, the dsRNA has a 3'-end overhang.
In a related aspect, the dsRNA has a blunt end.
In another aspect, the disclosure provides a double-stranded RNA (dsRNA) that downregulates the expression of a gene, the dsRNA comprising a sense strand and an antisense strand, a double-stranded region having from 25 to 40 base pairs, and wherein the last two nucleomonomers of the 3'-end of the antisense strand and the last nucleomonomer of the 3'-end of the sense strand are hydroxymethyl substituted nucleomonomers.
In another aspect, the disclosure provides a double-stranded RNA (dsRNA) that downregulates the expression of a gene, the dsRNA comprising a sense strand and an antisense strand, a double-stranded region having from 25 to 40 base pairs, and wherein one or more
hydroxymethyl substituted nucleomonomer(s) are at one or more of positions of the sense strand that inhibit processing of the dsRNA by a Dicer enzyme.
In a related aspect, one or more hydroxymethyl substituted nucleomonomer(s) are at one or more of positions 21 , 22 or 23 of the sense strand counting from the 5 '-end of the sense strand.
In a related aspect, one or more hydroxymethyl substituted nucleomonomer(s) are at one or more of positions 18, 19, 20 21 or 22 of the antisense strand counting from the 3'-end of the antisense strand.
In one aspect of the disclosure, the number of hydroxymethyl substituted
nucleomonomers in the antisense strand is 10. In other embodiments of the disclosure, the number of hydroxymethyl substituted nucleomonomer(s) in the antisense strand is 9, 8, 7, 6, 5, 4, 3, 2 or 1 , respectively.
In another aspect, all nucleomonomers of the antisense strand are hydroxymethyl substituted nucleomonomers.
In one aspect of the disclosure, all hydroxymethyl substituted nucleomonomers in the antisense strand are present in positions 1 , 2, 3, 4, 5, 6, 7, and/or 8, wherein the positions are counted from the 5' end of the antisense strand. Even more preferably, the hydroxymethyl substituted nucleomonomers in the antisense strand are present in positions 2, 3, 4, 5, 6, and/or 7, counted from the 5' end of the antisense strand or in the corresponding to the so-called seed region of a microRNA. In another aspect, the hydroxymethyl substituted nucleomonomers in the antisense strand are present in positions 4, 5, 6, 7 and/or 8, counted from the 5' end of the antisense strand. In another aspect, the hydroxymethyl substituted nucleomonomers in the antisense strand are present in positions 6, 7 and/or 8, counted from the 5' end of the antisense strand. In another aspect, the hydroxymethyl substituted nucleomonomers in the antisense strand are present in positions in the antisense strand that reduce the microRNA activity of the RNA compared to the same RNA without hydroxymethyl substituted nucleomonomers. Thus, presence of hydroxymethyl substituted nucleomonomers in the aforementioned regions may prevent the antisense strand from acting as a microRNA, which reduces off target effects when the antisense strand is intended to function as siRNA.
In a preferred embodiment, at least one hydroxymethyl substituted nucleomonomer is present in any one of positions 9, 10, 1 1, 12, 13, 14, 15, and/or 16, wherein the positions are counted from the 5 '-end of the antisense strand. Even more preferred is hydroxymethyl substituted nucleomonomers present in any one of positions 9, 10, 1 1 , 12, 13, 14, 15, and/or 16, wherein the positions are counted from the 5'end of the antisense strand. In another embodiment,
hydroxymethyl substituted nucleomonomers in the antisense strand is present in all of positions 9, 10, 1 1, 12, 13, 14, 15, and/or 16. In one embodiment, hydroxymethyl substituted
nucleomonomer are only present in regions 9, 10, 11, 12, 13, 14, 15, and/or 16 and not in the rest of the antisense strand.
Even more preferably, the hydroxymethyl substituted nucleomonomers in the antisense strand is present in position 9, 10, and/or 1 1, counted from the 5' end of the antisense strand, and preferably, not in the rest of the oligonucleotide. In another aspect, the hydroxymethyl substituted nucleomonomers in the antisense strand are present in positions in the antisense strand that enhance the microRNA activity of the RNA compared to the same RNA without hydroxymethyl substituted nucleomonomers. The presence of hydroxymethyl substituted nucleomonomers in the aforementioned regions may induce the antisense strand to act as a microRNA, i.e. ensure that the siRNA effect will be minimal and the microRNA effect much higher.
In another embodiment of the disclosure, the number of hydroxymethyl substituted nucleomonomers in the passenger strand of an siRNA complex of the disclosure is 10. In other embodiments of the disclosure, the number of hydroxymethyl substituted nucleomonomers in the passenger strand of a siRNA complex of the disclosure is 9, 8, 7, 6, 5, 4, 3, 2 or 1, respectively.
In another embodiment, all nucleomonomers of the passenger strand of a siRNA complex of the disclosure are hydroxymethyl substituted nucleomonomers.
In certain aspects, the sense (passenger strand) of a dsRNA comprises one or more hydroxymethyl substituted nucleomonomer(s). In certain aspects, the sense (passenger strand) of a dsRNA comprises 1 , 2, 3, 4, 5, 6, 7, 8, 9 or 10 hydroxymethyl substituted nucleomonomer(s). In certain aspects, the entire sense (passenger strand) of a dsRNA comprises hydroxymethyl substituted nucleomonomer(s).
In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 1, 2, 3, 4, 5, 6, 7, and/or 8 wherein the positions are counted from the 5'-end of the sense strand. In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 1 , 2, 3, and/or 4 wherein the positions are counted from the 5'-end of the sense strand. In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 1 , 2 and/or 3 wherein the positions are counted from the 5'-end of the sense strand. In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 5, 6, 7, and/or 8 wherein the positions are counted from the 5'-end of the sense strand. In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 7 and/or 8 wherein the positions are counted from the 5'-end of the
sense strand. In certain aspects, hydroxymethyl substituted nucleomonomers in the sense strand are present in positions in the sense strand of an RNA that reduce the RNAi activity of the sense strand of the RNA compared to the same RNA without hydroxymethyl substituted
nucleomonomers.
In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 9, 10, 1 1 , 12, 13, 14, 15, and/or 16 wherein the positions are counted from the 5 '-end of the sense strand. In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 9, 10, and/or 1 1 , wherein the positions are counted from the 5 '-end of the sense strand.
In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 and/or 32 wherein the positions are counted from the 5 '-end of the sense strand. In certain aspects, a hydroxymethyl substituted nucleomonomer in the sense strand is present in positions 1 , 2, 3, 4, 5, 6, 7, 8, 9 and/or 10, wherein the positions are counted from the 3 '-end of the sense strand.
In one embodiment, both the antisense strand and the passenger strand of a siRNA complex of the disclosure contain one or more hydroxymethyl substituted nucleomonomer(s).
In another embodiment, the RNA complex is single stranded and has no double stranded region.
In yet another embodiment, the RNA complex is single stranded but folds such that it contains one or more double stranded regions. Such embodiments are useful e.g. for mimicking microRNAs and their functions.
In yet another embodiment, the core double stranded region of a siRNA complex of the disclosure is shorter than 10 base pairs and thus comprises from one to nine base pairs.
In one embodiment of the disclosure, the core double stranded region of the RNA complex is comprised by more than two RNA strands.
In one embodiment of the disclosure, the core double stranded region of the RNA complex is comprised by three RNA strands.
In one aspect, the present disclosure provides an RNA complex capable of mediating nucleic acid modifications of a target nucleic acid. Such RNA complex may e.g. be a siRNA, microRNA or microRNA precursor (pre-microRNA).
The RNA complex of a siRNA complex of the disclosure comprises a core double stranded region comprising an antisense strand and a passenger strand that is hybridized to the antisense strand.
In another aspect, the instant disclosure provides for a method for reducing the expression of a human HRAS gene, comprising administering a nucleic acid as disclosed herein to a cell expressing a HRAS gene, wherein the nucleic acid reduces expression of the HRAS gene in the cell. In a related embodiment, the cell is a human cell.
In another aspect, the instant disclosure provides for a method for treating or managing a disease or condition in a subject associated, linked, and/or resulting from aberrant HRAS gene expression, comprising administering to the subject in need of treatment or management a nucleic acid comprising an antisense strand having a nucleic acid sequence selected from SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81 , 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278, and a sense strand complementary to the antisense strand, wherein the antisense strand and the sense strand can anneal to from 15 base pairs to 60 base pairs, wherein the nucleic acid reduces the expression of the HRAS gene thereby treating or managing the disease or condition. In another aspect, the instant disclosure provide for a method for treating or managing a disease or condition in a subject associated, linked, and/or resulting from aberrant HRAS gene expression, comprising administering to the subject in need of treatment or management a nucleic acid as disclosed herein, wherein the nucleic acid reduces the expression of the HRAS gene thereby treating or managing the disease or condition.
In a related embodiment, the disease or condition is selected from one or more hyperproliferative diseases or disorders, leukemia, cutaneous melanoma, adenocarcinoma, squamous cell carcinoma, Philadelphia chromosome-negative myeloproliferative disorder, myelodysplastic syndrome, transitional cell carcinoma, ovarian cancer, brain tumors, breast cancer, bladder cancer, lung cancer, kidney tumors, urinary tract tumors, pancreatic carcinoma, and colorectal adenoma; as well as one or more angiogenic diseases or disorders, hepatocellular carcinoma (HCC), NSCLC (lung nonsmall cell lung cancer), melanoma, colon cancer, prostate cancer, and glioblastoma.
In any of the aspects of this disclosure, some embodiments provide a nucleic acid comprising one or more 5-methyluridine (ribothymidine), a 2-thioribothymidine, or 2'-0-methyl- 5-methyluridine, deoxyuridine, locked nucleic acid (LNA) molecule, or a universal-binding nucleotide, or a G clamp. Exemplary universal-binding nucleotides include C-phenyl, C- naphthyl, inosine, azole carboxamide, l-P-D-ribofuranosyl-4-nitroindole, l-P-D-ribofuranosyl-5- nitroindole, l-P-D-ribofuranosyl-6-nitroindole, or l-P-D-ribofuranosyl-3-nitropyrrole. In some embodiments, the nucleic acid further comprises a 2'-sugar substitution, such as a 2'-0-methyl, 2'-0-methoxyethyl, 2'-0-2-methoxyethyl, 2'-0-allyl, or halogen (e.g., 2'-fluoro). In certain embodiments, the nucleic acid further comprises a terminal cap substituent on one or both ends
of one or more of the first strand, second strand, or third strand, such as independently an alkyl, abasic, deoxy abasic, glyceryl, dinucleotide, acyclic nucleotide, or inverted deoxynucleotide moiety. In other embodiments, the nucleic acid further comprises at least one modified internucleoside linkage, such as independently a phosphorothioate, chiral phosphorothioate, phosphorodithioate, phosphotriester, aminoalkylphosphotriester, methyl phosphonate, alkyl phosphonate, 3'-alkylene phosphonate, 5'-alkylene phosphonate, chiral phosphonate, phosphonoacetate, thiophosphonoacetate, phosphinate, phosphoramidate, 3'-amino
phosphoramidate, aminoalkylphosphoramidate, fhionophosphoramidate,
thionoalkylphosphonate, thionoalkylphosphotriester, selenophosphate, or boranophosphate linkage.
In any of the aspects disclosed herein, the RNA complex comprises a 2'-0-methyl nucleomonomer. In a related aspect, the RNA complex comprises from zero to twelve 2'-0- methyl nucleomonomer(s) (or 0, 1 , 2, 3, 4, 5, 6, 7, 8, 9, 10, 1 1 or 12 2'-0-mefhyl
nucleomonomer(s)). In a related aspect, the passenger strand of the RNA complex comprises from zero to twelve 2'-0-methyl nucleomonomer(s) (or 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 2'- O-mefhyl nucleomonomer(s)). In a related aspect, the guide strand of the RNA complex comprises from zero to six 2'-0-methyl nucleomonomer(s) (or 0, 1 , 2, 3, 4, 5 or 6 2'-0-methyl nucleomonomer(s)). In certain aspects, the hydroxymethyl substituted monomer is a 2'-0- methyl nucleomonomer.
In any of the aspects of this disclosure, some embodiments provide nucleic acid comprising an overhang of one to four nucleotides on at least one 3'-end that is not part of the gap. In any of the aspects of this disclosure, some embodiments provide a nucleic acid has a blunt end at one or both ends. In other embodiments, the 5'-terminal of the sense strand, antisense strand or both strands is a hydroxyl or a phosphate.
In one embodiment, the RNA complex may be a bifunctional RNA complex having two blunt-ends and a hydroxymethyl substituted nucleomonomer at position(s) 5, 6, 7, and/or 8 from the 5 '-end of each of the guide strand and passenger strand.
In one embodiment, the bifunctional RNA complex comprise two blunt-ends, a sense strand and a antisense strand, wherein the sense strand comprises an hydroxymethyl substituted nucleomonomer at position(s) 5, 6, 7, and/or 8 from the 5'-end of the sense strand, and the antisense strand comprises an hydroxymethyl substituted nucleomonomer at position(s) 5, 6, 7, and/or 8 from the 5 '-end of antisense strand, and wherein the sense strand is complementary to a first region of a target RNA and the antisense region is complementary to a second region of the target RNA, wherein the first region and the second region are non-overlapping regions of the
target RNA. In a related embodiment, the first and second regions of the target RNA partially overlap.
In one embodiment, the bifunctional RNA complex comprise two blunt-ends, a sense strand and a antisense strand, wherein the sense strand comprises an hydroxymethyl substituted nucleomonomer at position(s) 5, 6, 7, and/or 8 from the 5'-end of the sense strand, and the antisense strand comprises an hydroxymethyl substituted nucleomonomer at position(s) 5, 6, 7, and/or 8 from the 5 '-end of antisense strand, and wherein the sense strand is complementary to a first region of a first target RNA and the antisense region is complementary to a second region of a second target RNA, wherein the first target RNA and the second target RNA are different target RNAs, or have less than 95% homology, or 90% homology, or 85% homology, or 80% homology, or 75% homology, or 70% homology, or 65% homology, or 60% homology, or 55% homology or 50% homology. In a related embodiment, the first and second target RNAs are in the same cellular pathway.
In one aspect, the instant disclosure provide a method for selecting a subject for treatment with a nucleic acid that down regulates the expression of a v-Ha-ras Harvey rat sarcoma viral oncogene homolog gene (HRAS) mRNA comprising the steps of identifying a subject having cancer, determining whether a cancer cell in the subject expresses HRAS mRNA, determining whether the nucleic acid reduces HRAS mRNA expression in the cancer cell, selecting the subject having the cancer cell wherein HRAS mRNA expression was reduced by the nucleic acid for treatment with the nucleic acid.
In certain embodiments, the RAS-associated diseases or disorders include one or more hyperproliferative diseases or disorders, for example, leukemia, cutaneous melanoma, adenocarcinoma, squamous cell carcinoma, Philadelphia chromosome-negative
myeloproliferative disorder, myelodysplastic syndrome, transitional cell carcinoma, ovarian cancer, brain tumors, breast cancer, bladder cancer, lung cancer, kidney tumors, urinary tract tumors, pancreatic carcinoma, and colorectal adenoma; as well as one or more angiogenic diseases or disorders, hepatocellular carcinoma (HCC), NSCLC (lung nonsmall cell lung cancer), melanoma, colon cancer, prostate cancer, and glioblastoma.
In certain embodiments, the nucleic acid comprises an antisense strand having a nucleic acid sequence selected from SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81, 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278, and a sense strand complementary to the antisense strand, wherein the antisense strand and the sense strand can anneal to form 15 base pairs to 60 base pairs. In certain embodiments, the nucleic acid is a ribonucleic acid having a double-stranded region (dsRNA). In certain embodiments, the ribonucleic acid is a siRNA.
In certain embodiments, the antisense strand is 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 ,25 ,26, 27, 28, 29, 30, 31 , 32, 33, 34 ,35 ,36, 37, 38, 39, 40, 41 , 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, or 60 nucleomonomers in length. In other embodiments, the antisense strand is 18, 19, 20, 21 , 22, 23, 24 or 25 nucleomonomers in length.
In certain embodiments, the sense strand is a contiguous strand of nucleomonomers.
In certain embodiments, the sense strand has one or more nicks.
In certain embodiments, the sense strand has one or more gaps. In certain embodiments, the one or more gaps, independently for each occurrence, comprise from 1 , 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 , 12, 13, or 14 unpaired nucleomonomers.
In certain embodiments, the nucleic acid has a blunt end.
In certain embodiments, the nucleic acid further comprises a 3 '-end overhang.
In any one of the embodiments described herein, the nucleic acid further comprises at least one hydroxymethyl substituted nucleomonomer. In a related embodiment, the
hydroxymethyl substituted nucleomonomer is selected from:
wherein, R is selected from a hydrogen, a methyl group, C(l , 2 ,3 ,4 ,5 ,6, 7, 8, 9, 10) alkyl, cholesterol, naturally or non-naturally occurring amino acid, sugar, vitamin, fluorophore, polyamine and fatty acid, and wherein the Base is nucleobase or analog thereof.
In certain embodiments, one or both of the last two positions at the 3 '-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer. In
certain embodiments, one or both of the last two positions at the 3 '-end of the antisense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain embodiments, any one or more of the last three positions at the 5 '-end of the sense strand is occupied by the same or different hydroxymethyl substituted nucleomonomer. In certain embodiments, at least one hydroxymethyl substituted nucleomonomer is in a double-stranded region of the nucleic acid.
In anyone embodiment disclosed herein, the nucleic acid may contain one or more of nucleomonomers B or C shown below:
Synthesis of Nucleic Acid Molecules
Exemplary molecules of the instant disclosure are recombinantly produced, chemically synthesized, or a combination thereof. Oligonucleotides (e.g., certain modified oligonucleotides or portions of oligonucleotides lacking ribonucleotides) are synthesized using protocols known in the art, for example as described in Caruthers et al., Methods in Enzymol. 211:3-19, 1992; Thompson et ai, PCT Publication No. WO 99/54459, Wincott et al., Nucleic Acids Res.
23:2677-2684, 1995; Wincott et al., Methods Mol. Bio. 74:59, 1997; Brennan et al., Biotechnol Bioeng. 67:33-45, 1998; and Brennan, U.S. Patent No. 6,001,311. Synthesis of RNA, including certain dsRNA molecules and analogs thereof of this disclosure, can be made using the procedure as described in Usman et al., J. Am. Chem. Soc. 709:7845, 1987; Scaringe et al., Nucleic Acids Res. 75:5433, 1990; and Wincott et al., Nucleic Acids Res. 23:2677-2684, 1995; Wincott et al, Methods Mol. Bio. 74:59, 1997.
In certain embodiments, the nucleic acid molecules of the present disclosure can be synthesized separately and joined together post-synthetically, for example, by ligation (Moore et al., Science 256:9923, 1992; Draper et al., PCT Publication No. WO 93/23569; Shabarova et al., Nucleic Acids Res. 79:4247, 1991 ; Bellon et al., Nucleosides & Nucleotides 76:951, 1997;
Bellon et ai, Bioconjugate Chem. S:204, 1997), or by hybridization following synthesis or deprotection.
In further embodiments, dsRNAs of this disclosure that decrease expression of a HRAS gene by RNAi can be made as single or multiple transcription products expressed by a polynucleotide vector encoding one or more dsRNAs and directing their expression within host cells. In these embodiments the double-stranded portion of a final transcription product of the dsRNAs to be expressed within the target cell can be, for example, about 5 to about 40 bp, about 15 to about 24 bp, or about 25 to about 40 bp long. Within exemplary embodiments, double- stranded portions of dsRNAs, in which two or more strands pair up, are not limited to completely paired nucleotide segments, and may contain non-pairing portions due to a mismatch (the corresponding nucleotides are not complementary), bulge (lacking in the corresponding complementary nucleotide on one strand), overhang, or the like. Non-pairing portions can be contained to the extent that they do not interfere with dsRNA formation and function. In certain embodiments, a "bulge" may comprise 1 to 2 non-pairing nucleotides, and the double-stranded region of dsRNAs in which two strands pair up may contain from about 1 to 7, or about 1 to 5 bulges. In addition, "mismatch" portions contained in the double-stranded region of dsRNAs may include from about 1 to 7, or about 1 to 5 mismatches. In other embodiments, the double- stranded region of dsRNAs of this disclosure may contain both bulge and mismatched portions in the approximate numerical ranges specified herein.
A dsRNA or analog thereof of this disclosure may be further comprised of a nucleotide, non-nucleotide, or mixed nucleotide/non-nucleotide linker that joins the sense region of the dsRNA to the antisense region of the dsRNA. In one embodiment, a nucleotide linker can be a linker of more than about 2 nucleotides length up to about 10 nucleotides in length. In another embodiment, the nucleotide linker can be a nucleic acid aptamer.
A non-nucleotide linker may be comprised of an abasic nucleotide, polyether, polyamine, polyamide, peptide, carbohydrate, lipid, polyhydrocarbon, or other polymeric compounds {e.g., polyethylene glycols such as those having between 2 and 100 ethylene glycol units). Specific examples include those described by Seela and Kaiser, Nucleic Acids Res. 75:6353, 1990, and Nucleic Acids Res. 75:31 13, 1987; Cload and Schepartz, /. Am. Chem. Soc. 775:6324, 1991 ; Richardson and Schepartz, J. Am. Chem. Soc. 113.5109, 1991 ; Ma et al., Nucleic Acids Res. 27:2585, 1993, and Biochemistry 32: 1751 , 1993; Durand et al., Nucleic Acids Res. 78:6353, 1990; McCurdy et al, Nucleosides & Nucleotides 70:287, 1991; Jaschke et al., Tetrahedron Lett. 34:301 , 1993; Ono et al., Biochemistry 30:9914, 1991 ; Arnold et al, PCT Publication No.
WO 89/02439; Usman et al, PCT Publication No. WO 95/06731 ; Dudycz et al, PCT
Publication No. WO 95/1 1910 and Ferentz and Verdine, J. Am. Chem. Soc. 773:4000, 1991. The synthesis of a dsRNA molecule of this disclosure, which can be further modified, comprises: (a) synthesis of a first (antisense) strand and synthesis of a second (sense) strand and a third (sense) strand that are each complementary to non-overlapping regions of the first strand; and (b) annealing the first, second and third strands together under conditions suitable to obtain a dsRNA molecule. In another embodiment, synthesis of the first, second and third strands of a dsRNA molecule is by solid phase oligonucleotide synthesis. In yet another embodiment, synthesis of the first, second, and third strands of a dsRNA molecule is by solid phase tandem oligonucleotide synthesis.
Chemically synthesizing nucleic acid molecules with substitutions or modifications
(base, sugar, phosphate, or any combination thereof) can prevent their degradation by serum ribonucleases, which may lead to increased potency. See, e.g., Eckstein et al, PCT Publication No. WO 92/07065; Perrault et al., Nature 344:565, 1990; Pieken et al., Science 253:314, 1991; Usman and Cedergren, Trends in Biochem. Sci. 7:334, 1992; Usman et al., Nucleic Acids Symp. Ser. 37: 163, 1994; Beigelman et al, J. Biol. Chem. 270:25702, 1995; Burgin et al, Biochemistry 35: 14090, 1996; Burlina et al, Bioorg. Med. Chem. 5: 1999, 1997; Thompson et al, Karpeisky et al, Tetrahedron Lett. 39: 1131, 1998; Earnshaw and Gait, Biopolymers (Nucleic Acid Sciences) 48:39-55, 1998; Verma and Eckstein, Annu. Rev. Biochem. 67:99-134, 1998; Herdewijn, Antisense Nucleic Acid Drug Dev. 70:297, 2000; Kurreck, Eur. J. Biochem. 270: 1628, 2003; Dorsett and Tuschl, Nature Rev. Drug Discov. 3:318, 2004; PCT Publication Nos.
WO 91/03162; WO 93/15187; WO 97/26270; WO 98/13526; U.S. Patent Nos. 5,334,71 1 ;
5,627,053; 5,716,824; 5,767, 264; 6,300,074. Each of the above references discloses various substitutions and chemical modifications to the base, phosphate, or sugar moieties of nucleic acid molecules, which can be used in the dsRNAs described herein. For example,
oligonucleotides can be modified at the sugar moiety to enhance stability or prolong biological activity by increasing nuclease resistance. Representative sugar modifications include 2'-amino, 2'-C-allyl, 2'-fluoro, 2'-0-methyl, 2'-0-allyl, or 2'-H. Other modifications to enhance stability or prolong biological activity can be internucleoside linkages, such as phosphorothioate, or base- modifications, such as locked nucleic acids (see, e.g., U.S. Patent Nos. 6,670,461; 6,794,499; 6,268,490), or 5-methyluridine or 2'-0-methyl-5-methyluridine in place of uridine (see, e.g., U.S. Patent Application Publication No. 2006/0142230). Hence, dsRNA molecules of the instant disclosure can be modified to increase nuclease resistance or duplex stability while substantially retaining or having enhanced RNAi activity as compared to unmodified dsRNA.
In one embodiment, this disclosure features substituted or modified dsRNA molecules, such as phosphate backbone modifications comprising one or more phosphorothioate, phosphorodithioate, methylphosphonate, phosphotriester, morpholino, amidate carbamate, carboxymethyl, acetamidate, polyamide, sulfonate, sulfonamide, sulfamate, formacetal, thioformacetal, or alkylsilyl substitutions. For a review of oligonucleotide backbone modifications, see Hunziker and Leumann, Nucleic Acid Analogues: Synthesis and Properties, in Modern Synthetic Methods, VCH, 331-417, 1995; and Mesmaeker et al, ACS, 24-39, 1994.
In another embodiment, a conjugate molecule can be optionally attached to a dsRNA or analog thereof that decreases expression of a HRAS gene by RNAi. For example, such conjugate molecules may be polyethylene glycol, human serum albumin, polyarginine, Gln-Asn polymer, or a ligand for a cellular receptor that can, for example, mediate cellular uptake (e.g., HIV TAT, see Vocero-Akbani et al., Nature Med. 5:23, 1999; see also U.S. Patent Application Publication No. 2004/0132161).. Examples of specific conjugate molecules contemplated by the instant disclosure that can be attached to a dsRNA or analog thereof of this disclosure are described in Vargeese et al., U.S. Patent Application Publication No. 2003/0130186, and U.S. Patent Application Publication No. 2004/01 10296.
In another embodiment, a conjugate molecule is covalently attached to a dsRNA or analog thereof that decreases expression of a HRAS gene by RNAi via a biodegradable linker. In certain embodiments, a conjugate molecule can be attached at the 3'-end of either the sense strand, the antisense strand, or both strands of a dsRNA molecule provided herein. In another embodiment, a conjugate molecule can be attached at the 5'-end of either the sense strand, the antisense strand, or both strands of the dsRNA or analog thereof. In yet another embodiment, a conjugate molecule is attached at both the 3'-end and 5'-end of either the sense strand, the antisense strand, or both strands of a dsRNA molecule, or any combination thereof. In further embodiments, a conjugate molecule of this disclosure comprises a molecule that facilitates delivery of a dsRNA or analog thereof into a biological system, such as a cell. A person of skill in the art can screen dsRNA of this disclosure having various conjugates to determine whether the dsRNA-conjugate possesses improved properties (e.g., pharmacokinetic profiles, bioavailability, stability) while maintaining the ability to mediate RNAi in, for example, an animal model as described herein or generally known in the art.
The hydroxymethyl substituted RNA oligonucleotides (= RNA strands) and RNA complexes can be synthesised using phosphoramidite derivatives using the standard techniques for RNA synthesis. Methods for synthesis of hydroxymethyl substituted RNA oligonucleotides
may be found in PCT patent application PCT/US2008/064417; which is hereby incorporated by reference in its entirety.
DILA2 amino acid nanoparticle-forming compounds
Nanoparticle compositions of this disclosure may include one or more DILA2 amino acid compounds which are disclosed in US 2008-0317839 Al .
DILA2 amino acid compounds are synthetic organic compounds that may form nanoparticle structures under certain conditions. DILA2 amino acid compounds may be formed by substituting a delivery-enhancing or lipophilic tail at either the N-terminus or the C-terminus of an amino acid, or at both termini. In some embodiments, the amino acid core may include one or more amino acids, or may be a peptide of 2-20 amino acid residues.
DILA2 amino acid compounds can be cationic or non-cationic, where non-cationic includes neutral and anionic. As used herein, the physical state or ionicity of a species refers to an environment having pH about 7, unless otherwise specified.
In some aspects, DILA2 amino acid compounds may provide delivery of a therapeutic agent in a releasable form. Releasable forms and compositions are designed to provide sufficient uptake of an agent by a cell to provide a therapeutic effect.
Releasable forms include DILA2 amino acid compounds that bind and release an active agent. In some embodiments, release of the active agent may be provided by an acid-labile linker.
Examples of acid-labile linkers include linkers containing an orthoester group, a hydrazone, a cis-acetonyl, an acetal, a ketal, a silyl ether, a silazane, an imine, a citriconic anhydride, a maleic anhydride, a crown ether, an azacrown ether, a thiacrown ether, a dithiobenzyl group, a cis-aconitic acid, a cis-carboxylic alkatriene, methacrylic acid, and mixtures thereof.
Examples of acid-labile groups and linkers are given in U.S. Patent Nos. 7,098,032; 6,897,196; 6,426,086; 7,138,382; 5,563,250; and 5,505,931.
Releasable forms of compounds and compositions of this disclosure include molecules that bind an active agent and discharge a moiety that assists in release of the agent. In some embodiments, a DILA2 amino acid compound may include a group which releases a small molecule such as ethanol that assists in delivering an agent to a cell. A DILA2 amino acid compound may bind an active agent and, subsequent to contact with a cell, or subsequent to transport within a biological compartment having a local pH lower than physiological pH, be
hydrolyzed in an acidic environment to release ethanol to assist in delivery of the agent. In some embodiments, a small molecule such as ethanol, which assists in delivery of the agent, may be bound to a lipophilic component.
In some embodiments, a DILA2 amino acid compound may be admixed with a compound that releases a small molecule such as ethanol to assists in delivering an agent to a cell.
Releasable forms of compounds and compositions of this disclosure include DILA2 amino acid compounds which may bind an active agent and, subsequent to contact with a cell, or subsequent to transport within a biological compartment having a local pH lower than physiological pH, be modulated in an acidic environment into a cationic form to assist in release of the agent.
In some embodiments, a DELA2 amino acid compound may bind an active agent, and may be admixed with a compound that can be modulated in an acidic environment into a cationic form to assist in release of an active agent.
Examples of hydrolysable and modulatable groups are given in U.S. Patent Nos.
6,849,272; 6,200,599; as well as Z. H. Huang and F. C. Szoka, "Bioresponsive nanoparticles and their use for macromolecular delivery," in: G. Gregoriadis (ed.), Nanoparticle Technology, 3rd ed. (CRC Press 2006).
In some embodiments, releasable forms of compounds and compositions of this disclosure include DILA2 amino acid compounds which can bind an active agent, and may be admixed with a lipid or compound that can be modulated in an acidic environment into a neutral form to assist in release of an active agent. The acidic environment may be entered subsequent to contact with a cell, or subsequent to transport within a biological compartment having a local pH lower than physiological pH.
Examples of compounds which are modulatable from anionic to neutral forms include cholesteryl hemisuccinate (CHEMS) as described in U.S. Patent Nos. 6,897,196; 6,426,086; and 7,108,863. In some examples, CHEMS exhibits pH sensitive polymorphism as described in Cullis, 1463 Biochimica et Biophysica Acta 107-14 (2000).
In some embodiments, releasable forms of compounds and compositions of this disclosure include DILA2 amino acid compounds which can bind an active agent, and may be admixed with a pH-sensitive polymeric material.
Examples of pH-sensitive polymeric materials are given in U.S. Patent No. 6,835,393. In some embodiments, release of the active agent may be provided by an enzyme- cleavable peptide.
In some aspects, this disclosure provides a range of DILA2 amino acid compounds as shown in Formula I:
R3-(C=0)-Xaa-Z-R4 Formula I wherein
Xaa is any D- or L-amino acid residue having the general formula -NRN-CR1R2-(C=0)-, or a peptide of 2-20 amino acid residues, wherein
R1 is a non-hydrogen, substituted or unsubstituted side chain of an amino acid; R2 is hydrogen, or an organic group consisting of carbon, oxygen, nitrogen, sulfur, and hydrogen atoms, and having from 1 to 20 carbon atoms, or C( 1 - 5)alkyl, cycloalkyl, cycloalkylalkyl, C(3-5)alkenyl, C(3-5)alkynyl, C(l- 5)alkanoyl, C(l -5)alkanoyloxy, C(l -5)alkoxy, C(l-5)alkoxy-C(l -5)alkyl, C(l-5)alkoxy-C(l-5)alkoxy, C( l-5)alkyl-amino-C(l-5)alkyl-, C(l- 5)dialkyl-amino-C(l-5)alkyl-, nitro-C(l-5)alkyl, cyano-C(l-5)alkyl, aryl-C(l- 5)alkyl, 4-biphenyl-C(l-5)alkyl, carboxyl, or hydroxyl,
RN is hydrogen, or an organic group consisting of carbon, oxygen, nitrogen, sulfur, and hydrogen atoms, and having from 1 to 20 carbon atoms, or C(l- 5)alkyl, cycloalkyl, cycloalkylalkyl, C(3-5)alkenyl, C(3-5)alkynyl, C(l- 5)alkanoyl, C(l-5)alkanoyloxy, C(l-5)alkoxy, C(l-5)alkoxy-C(l-5)alkyl, C(l-5)alkoxy-C(l-5)alkoxy, C(l-5)alkyl-amino-C(l-5)alkyl-, C(l- 5)dialkyl-amino-C(l-5)alkyl-, nitro-C(l -5)alkyl, cyano-C(l-5)alkyl, aryl-C(l- 5)alkyl, 4-biphenyl-C(l -5)alkyl, carboxyl, or hydroxyl,
R3 is a lipophilic tail derived from a naturally-occurring or synthetic phospholipid,
glycolipid, triacylglycerol, glycerophospholipid, sphingolipid, ceramide, sphingomyelin, cerebroside, or ganglioside; or a substituted or unsubstituted C(3- 22)alkyl, C(6- 12)cycloalkyl, C(6-12)cycloalkyl-C(3-22)alkyl, C(3-22)alkenyl, C(3- 22)alkynyl, C(3-22)alkoxy, or C(6- 12)alkoxy-C(3-22)alkyl; or a lipophilic tail of any other naturally-occurring or synthetic lipid, or a lipophilic tail of any one of the lipids described hereinbelow, and may contain a steroid;
R4 is a lipophilic tail derived from a naturally-occurring or synthetic phospholipid,
glycolipid, triacylglycerol, glycerophospholipid, sphingolipid, ceramide, sphingomyelin, cerebroside, or ganglioside; or substituted or unsubstituted C(3- 22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl-C(3-22)alkyl, C(3-22)alkenyl, C(3- 22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy-C(3-22)alkyl; or a lipophilic tail of any
other naturally-occurring or synthetic lipid, or a lipophilic tail of any one of the lipids described hereinbelow, and may contain a steroid;
Z is NH, O, S, -CH2S-, -CH2S(0)-, or an organic linker consisting of 1-40 atoms selected from hydrogen, carbon, oxygen, nitrogen, and sulfur atoms;
and salts thereof.
In some embodiments, R3 is independently a substituted or unsubstituted C(6-22)alkyl or C(6-22)alkenyl; R4 is independently a substituted or unsubstituted C(6-22)alkyl or C(6- 22)alkenyl.
The residue Xaa may be a D- or L-stereocenter.
In some embodiments, R1 is a non-hydrogen, substituted or unsubstituted side chain of an amino acid wherein a substituent of a side chain is an organic group consisting of 1 to 40 atoms selected from hydrogen, carbon, oxygen, nitrogen, and sulfur atoms.
In some embodiments, Z is an alkyl or an organic linker synthetic polymer such as a polyethylene glycol chain (PEG), or a PEG copolymer such as PEG-polyurethane or PEG- polypropylene. See, e.g., J. Milton Harris, Poly(ethylene glycol) chemistry: biotechnical and biomedical applications (1992).
In some embodiments, this disclosure provides a range of DILA2 amino acid compounds as shown in Formula I above wherein:
Xaa is any D- or L-amino acid having the general formula -NRN-CR'R2-(C=0)-, wherein R1 is a non-hydrogen, substituted or unsubstituted basic side chain of an amino acid;
R2 is hydrogen, or C(l-5)alkyl,
RN is hydrogen, or C(l-5)alkyl,
R3 is a lipophilic tail derived from a naturally-occurring or synthetic phospholipid,
glycolipid, triacylglycerol, glycerophospholipid, sphingolipid, ceramide, sphingomyelin, cerebroside, or ganglioside; or a substituted or unsubstituted C(3- 22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl-C(3-22)alkyl, C(3-22)alkenyl, C(3- 22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy-C(3-22)alkyl; or a lipophilic tail of any other naturally-occurring or synthetic lipid, or a lipophilic tail of any one of the lipids described hereinbelow, and may contain a steroid;
R4 is a lipophilic tail derived from a naturally-occurring or synthetic phospholipid, glycolipid, triacylglycerol, glycerophospholipid, sphingolipid, ceramide, sphingomyelin, cerebroside, or ganglioside; or substituted or unsubstituted C(3- 22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl-C(3-22)alkyl, C(3-22)alkenyl, C(3-
22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy-C(3-22)alkyl; or a lipophilic tail of any other naturally-occurring or synthetic lipid, or a lipophilic tail of any one of the lipids described hereinbelow, and may contain a steroid;
Z is NH, O, S, -CH2S-, -CH2S(O)-, or an organic linker consisting of 1-40 atoms selected from hydrogen, carbon, oxygen, nitrogen, and sulfur atoms.
In some embodiments, this disclosure provides a range of DILA2 amino acid compounds shown in Formula I above wherein:
Xaa is any D- or L-amino acid having the general formula -NRN-CR'R2-(C=0)-, wherein R1 is a non-hydrogen, substituted or unsubstituted basic side chain of an amino acid;
R2 is hydrogen, or C(l-5)alkyl,
RN is hydrogen, or C(l-5)alkyl,
R3 is a substituted or unsubstituted C(3-22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl- C(3-22)alkyl, C(3-22)alkenyl, C(3-22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy- C(3-22)alkyl;
R4 is a substituted or unsubstituted C(3-22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl- C(3-22)alkyl, C(3-22)alkenyl, C(3-22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy- C(3-22)alkyl;
Z is NH, O, S, -CH2S-, -CH2S(0)-, or an organic linker consisting of 1-40 atoms selected from hydrogen, carbon, oxygen, nitrogen, and sulfur atoms.
In some embodiments, this disclosure provides a range of DILA2 amino acid compounds shown in Formula I above wherein:
Xaa is any D- or L-arhino acid having the general formula -NRN-CR1R2-(C=0)-, wherein R1 is a non-hydrogen, substituted or unsubstituted basic side chain of an amino acid;
R2 is hydrogen, or C(l-5)alkyl,
RN is hydrogen, or C(l-5)alkyl,
R3 is a substituted or unsubstituted C(3-22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl- C(3-22)alkyl, C(3-22)alkenyl, C(3-22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy- C(3-22)alkyl;
R4 is a substituted or unsubstituted C(3-22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl- C(3-22)alkyl, C(3-22)alkenyl, C(3-22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy- C(3-22)alkyl;
Z is NH.
In some embodiments, this disclosure provides a range of DILA2 amino acid compounds as shown in Formula I above wherein:
Xaa is any D- or L-amino acid having the general formula -NRN-CR1R2-(C=O)-, wherein R1 is a non-hydrogen, substituted or unsubstituted basic side chain of an amino acid;
R2 is hydrogen, or C(l-5)alkyl,
RN is hydrogen, or C(l-5)alkyl,
R3 is a substituted or unsubstituted C(3-22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl- C(3-22)alkyl, C(3-22)alkenyl, C(3-22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy- C(3-22)alkyl;
R4 is a substituted or unsubstituted C(3-22)alkyl, C(6-12)cycloalkyl, C(6-12)cycloalkyl- C(3-22)alkyl, C(3-22)alkenyl, C(3-22)alkynyl, C(3-22)alkoxy, or C(6-12)alkoxy- C(3-22)alkyl;
Z is O.
Cationic DILA2 amino acid compounds can be prepared where, for example, Xaa has a basic side chain. Examples of amino acids having a basic side chain include arginine (Arg), homoarginine (homoArg) (side chain -(CH2)4NH(C=NH)NH2), norarginine (norArg) (side chain -(CH2)2NH(C=NH)NH2), nor-norarginine (nornorArg) (side chain -(CH2)NH(C=NH)NH2), ornithine, lysine, homolysine, histidine, 1-methylhistidine, pyridylalanine (Pal), asparagine, N-ethylasparagine, glutamine, and 4-aminophenylalanine,.and side chain modified derivatives thereof.
Anionic DILA2 amino acid compounds can be prepared where, for example, Xaa is glutamate, aspartate, or succinylated serine.
Cationic DILA2 amino acid compounds can be prepared where, for example, Xaa is norArginine, pyridylanine, histidine, lysine, ornithine, diaminobutryic acid, diaminopropionic acid, and methylated forms thereof (e.g., mono-methylated, di-methylated, or tri-methylated).
Cationic and anionic D1LA2 amino acid compounds can also be prepared where the amino acid side chain contains an ionizable group or substituent.
Non-cationic or neutral DILA2 amino acid compounds can be prepared where, for example, Xaa is leucine, valine, alanine, or serine.
In some embodiments, Xaa is NG-methylarginine, symmetric or asymmetric NG,NG- dimethylarginine, NG-methyl-homoarginine, symmetric or asymmetric NG,NG-dimethyl- homoarginine, NG-methyl-norarginine, symmetric or asymmetric NG,NG-dimethyl-norarginine, or NG-methyl-nor-norarginine, symmetric or asymmetric NG NG-dimethyl-nor-norarginine.
In some embodiments, Xaa is NG-ethylarginine, symmetric or asymmetric NG,NG- diethylarginine, NG-ethyl-homoarginine, symmetric or asymmetric NG,NG-diethyl-homoarginine, NG-ethyl-norarginine, symmetric or asymmetric NG,NG-diethyl-norarginine, or NG-ethyl-nor- norarginine, symmetric or asymmetric NG,NG-diethyl-nor-norarginine.
In certain embodiments, Xaa is NG-alkylarginine, symmetric or asymmetric NG,NG- dialkylarginine, NG-alkyl-homoarginine, symmetric or asymmetric NG,NG-dialkyl-homoarginine, NG-alkyl-norarginine, symmetric or asymmetric NG,NG-dialkyl-norarginine, or NG-alkyl-nor- norarginine, symmetric or asymmetric NG,NG-dialkyl-nor-norarginine.
In some embodiments, Xaa is an amino acid having a guanidine- or amidine-containing side chain. For example, the side chain of the Xaa residue may contain a group such as guanido, amidino, dihydroimidazole, 4-guanido-phenyl, 4-amidino-phenyl, N-amidino-piperidine, N- amidino-piperazine, 4,5-dihydroimidazole, 2-(N-amidino)-pyrrolidinyl, or 4-[(2- aminopyrimidinyl)]ethyl.
Examples of Xaa side chains include the following structures, as well as their salt forms:
Examples of a substituted side chain of an amino acid suitable for a releasable form of a DILA2 amino acid compound include a releasing functional group having a pKa from about 5 to about 7.5, or from about 6 to about 7. In general, a releasing functional group which is a weak
base may exhibit a predominant neutral form at a local pH above pKa, and may exhibit a predominant ionic form at a local pH below pKa. A releasing functional group which is a weak acid may exhibit an ionic form at a local pH above pKa, and may exhibit a neutral form at a local pH below pKa. See, e.g., P. Heinrich Stahl, Handbook of Pharmaceutical Salts (2002).
In some embodiments, Xaa may have a side chain containing a functional group having a pKa from 5 to 7.5.
Examples of a substituted side chain of an amino acid suitable for a releasable form of a DILA2 amino acid compound include 1-methylhistidine.
Examples of a substituted side chain of an amino acid suitable for a releasable form of a DILA2 amino acid compound include 3,5-diiodo-tyrosine.
Examples of a substituted side chain of an amino acid suitable for a releasable form of a DILA2 amino acid compound include the following structures:
Examples of DILA2 amino acid compounds include the structures:
Examples of a substituent on a side chain of an amino acid suitable for a releasable form of a DILA2 amino acid compound include releasing functional groups derived from 3,5-diiodo- tyrosine, 1 -methylhistidine, 2-Methylbutanoic acid, 2-o-Anisylpropanoic acid, meso-Tartaric acid, 4,6-Dimethylpyrimidinamine, /7-Phthalic acid, Creatinine, Butanoic acid, NN-Dimethyl-1- naphthylamine, Pentanoic acid, 4-Methylpentanoic acid, N-Methylaniline, 1 ,10-Phenanthroline, 3-Pyridinecarboxylic acid, Hexanoic acid, Propanoic acid, 4-Animobenzoic acid, 2- Methylpropanoic acid, Heptanoic acid, Octanoic acid, Cyclohexanecarboxylic acid, Quinoline, 3-Quinolinamine, 2-Aminobenzoic acid, 4-Pyridinecarboxylic acid, Nonanic acid, Melamine, 8- Quinolinol, Trimethylacetic acid, 6-Methoxyquinoline, 4-(Methylamino)benzoic acid, p- Methylaniline, 3-(Methylamino)benzoic acid, Malic acid, N-Ethylaniline, 2-Benzylpyridine, 3,6-Dinitrophenol, N,N-Dimethylaniline, 2,5-Dimethylpiperazine, p-Phenetidine,
5-Methylquinoline, 2-Phenylbenzimidazole, Pyridine, Picolinic acid, 3,5-Diiodityrosine, p-Anisidine, 2-(Methylamino)benzoic acid, 2-Thiazolamine, Glutaric acid, Adipic acid, Isoquinoline, Itaconic acid, o-Phthalic acid, Benzimidazole, Piperazine, Heptanedioic acid, Acridine, Phenanthridine, Succinic acid, Methylsuccinic acid, 4-Methylquinoline,
3-Methylpyridine, 7-Isoquinolinol, Malonic acid, Methymalonic acid, 2-Methylquinoline, 2-Ethylpyridine, 2-Methylpyridine, 4-Methylpyridine, Histamine, Histidine, Maleic acid, cis-1,2- Cyclohexanediamine, 3,5-Dimethylpyridine, 2-Ethylbenzimidazole, 2-Methylbenzimidazole, Cacodylic acid, Perimidine, Citric acid, Isocitric acid, 2,5-Dimethylpyridine, Papaverine, 6- Hydroxy-4-methylpteridine, L-Thyroxine, 3,4-Dimethylpyridine, Methoxypyridine, trans- 1,2- Cyclohexanediamine, 2,5-Pyridinediamine, M -Methylhistidine, /-3-Methylhistidine, 2,3- Dimethylpyridine, Xanthopterin, 1 ,2-Propanediamine, N,N-Diethylaniline, Alloxanic acid, 2,6-Dimethylpyridine, L-Carnosine, 2-Pyridinamine, N-b-Alanylhistidine, Pilocarpine,
1-Methylimidazol, 1H-Imidazole, 2,4-Dimethylpyridine, 4-Nitrophenol, 2-Nitrophenol, Tyrosineamide, 5-Hydoxxyquinazoline, 1,1-Cyclopropanedicarboxylic acid,
2,4,6-Trimethylpyridine, Veronal, 2,3-Dichlorophenol, 1 ,2-Ethanediamine, 1-Isoquinolinamine, and combinations thereof.
In some embodiments, a range of DILA2 amino acid compounds corresponding to Formula I are represented by the structures
Structure 1A and
Structure IB where R1, R2, RN, R3, and R4 are defined as above.
In some embodiments, R3 and R4 are independently selected lipophilic tails which impart sufficient lipophilic character or lipophilicity, such as defined by water/octanol partitioning, to provide delivery across a membrane or uptake by a cell. These tails provide, when used in a DILA2 amino acid compound, an amphipathic molecule. Lipophilic tails may be derived from phospholipids, glycolipids, triacylglycerols, glycerophospholipids, sphingolipids, ceramides, sphingomyelins, cerebrosides, or gangliosides, among others, and may contain a steroid.
In certain embodiments, R3 and R4 may independently be a lipophilic tail having a glycerol backbone.
In some embodiments, R3 and R4 may independently be ClOalkyl, CI lalkyl, C12alkyl, C alkyl, CHalkyl, C15alkyl, C16alkyl, C17alkyl, C18alkyl, C19alkyl, C20alkyl, C21alkyl, or C22alkyl.
In some embodiments, R3 and R4 may independently be lipophilic tails having one of the following structures:
In the figure above, X represents the atom of the tail that is directly attached to the amino acid residue terminus, and is counted as one of the atoms in the numerical designation, for example, "18:3." In some embodiments, X may be a carbon, nitrogen, or oxygen atom.
In some embodiments, R3 and R4 may independently be lipophilic tails having one of the following structures:
Phytanoyl
In some embodiments, R3 and R4 are independently selected lipophilic tails which may contain a cholesterol, a sterol, or a steroid such as gonanes, estranes, androstanes, pregnanes,
cholanes, cholestanes, ergostanes, campestanes, poriferastanes, stigmastanes, gorgostanes, lanostanes, cycloartanes, as well as sterol or zoosterol derivatives of any of the foregoing, and their biological intermediates and precursors, which may include, for example, cholesterol, lanosterol, stigmastanol, dihydrolanosterol, zymosterol, zymostenol, desmosterol,
7-dehydrocholesterol, and mixtures and derivatives thereof.
In certain embodiments, R3 and R4 may independently be derived from fatty acid-like tails such as tails from myristic acid (C14:0)alkenyl, palmitic acid (C16:0)alkenyl, stearic acid (C18:0)alkenyl, oleic acid (C18: l, double bond at carbon 9)alkenyl, linoleic acid (C18:2, double bond at carbon 9 or 12)alkenyl, linonenic acid (C18:3, double bond at carbon 9, 12, or
15)alkenyl, arachidonic acid (C20:4, double bond at carbon 5, 8, 1 1 , or 14)alkenyl, and eicosapentaenoic acid (C20:5, double bond at carbon 5, 8, 1 1, 14, or 17)alkenyl. Other examples of fatty acid-like tails are found at Donald Voet and Judith Voet, Biochemistry, 3rd Edition (2005), p. 383.
In some embodiments, R3 and R4 may independently be derived from an isoprenoid. As used herein, the term "amino acid" includes naturally-occurring and non-naturally occurring amino acids. Thus, a DILA2 amino acid compound can be made from a genetically encoded amino acid, a naturally occurring non-genetically encoded amino acid, or a synthetic amino acid.
Examples of amino acids include Ala, Arg, Asn, Asp, Cys, Gin, Glu, Gly, His, Ue, Leu, Lys, Met, Phe, Pro, Ser, Thr, Trp, Tyr, and Val.
Examples of amino acids include azetidine, 2-aminooctadecanoic acid, 2-aminoadipic acid, 3-aminoadipic acid, 2,3-diaminopropionic acid, 2-aminobutyric acid, 4-aminobutyric acid, 2,3-diaminobutyric acid, 2,4-diaminobutyric acid, 2-aminoisobutyric acid, 4-aminoisobutyric acid, 2-aminopimelic acid, 2,2'-diaminopimelic acid, 6-aminohexanoic acid, 6-aminocaproic acid, 2-aminoheptanoic acid, desmosine, ornithine, citrulline, N-methylisoleucine, norleucine, tert-leucine, phenylglycine, t-butylglycine, N-methylglycine, sacrosine, N-ethylglycine, cyclohexylglycine, 4-oxo-cyclohexylglycine, N-ethylasparagine, cyclohexylalanine, t- butylalanine, naphthylalanine, pyridylalanine, 3-chloroalanine, 3-benzothienylalanine, 4- halophenylalanine, 4-chlorophenylalanine, 2-fluorophenylalanine, 3-fluorophenylalanine, 4-fluorophenylalanine, penicillamine, 2-thienylalanine, methionine, methionine sulfoxide, homoarginine, norarginine, nor-norarginine, N-acetyllysine, 4-aminophenylalanine,
N-methylvaline, homocysteine, homoserine, hydroxylysine, allo-hydroxylysine, 3- hydroxyproline, 4-hydroxyproline, isodesmosine, allo-isoleucine, 6-N-methyllysine, norvaline,
O-allyl-serine, O-allyl-threonine, alpha-aminohexanoic acid, alpha-aminovaleric acid, and pyroglutamic acid.
As used herein, the term "amino acid" includes alpha- and beta- amino acids.
Other amino acid residues can be found in Fasman, CRC Practical Handbook of Biochemistry and Molecular Biology, CRC Press, Inc. (1989).
In general, a compound may contain one or more chiral centers. Compounds containing one or more chiral centers may include those described as an "isomer," a "stereoisomer," a "diastereomer," an "enantiomer," an "optical isomer," or as a "racemic mixture." Conventions for stereochemical nomenclature, for example the stereoisomer naming rules of Cahn, Ingold and Prelog, as well as methods for the determination of stereochemistry and the separation of stereoisomers are known in the art. See, for example, Michael B. Smith and Jerry March, March's Advanced Organic Chemistry, 5th edition, 2001. The compounds and structures of this disclosure are meant to encompass all possible isomers, stereoisomers, diastereomers, enantiomers, and/or optical isomers that would be understood to exist for the specified compound or structure, including any mixture, racemic or otherwise, thereof.
Examples of DILA2 amino acid compounds include R3-(C=0)-Arg-NH-R4 wherein Arg is D- or L-arginine, and R3 and R4 are independently alkyl or alkenyl.
Examples of DILA2 amino acid compounds include the following structures:
Compound 1
Compound 2
Examples of DELA2 amino acid compounds include the following structures:
Compound 3
Compound 4
Compound 5
Compound 6
Examples of DILA2 amino acid compounds include R3-(C=0)-norArg-NH-R4 wherein norArg is D- or L-norarginine, and R3 and R4 are independently alkyl or alkenyl.
Examples of DILA2 amino acid compounds include the following structures:
Examples of DILA2 amino acid compounds include R3-(C=0)-nornorArg-NH-R4 (or R3-(C=0)-Diaminoproprionic Acid-NH-R4 wherein R3 and R4 are independently alkyl or alkenyl) wherein nornorArg is D- or L-nor-norarginine, and R3 and R4 are independently alkyl such as heptyl, octyl, nonyl, decyl, and undecyl.
Examples of DILA2 amino acid compounds include the following structures:
Compound 20
Examples of DILA2 amino acid compounds include R3-(C=0)-homoArg-NH-R4 wherein homoArg is D- or L-homoarginine, and R3 and R4 are independently alkyl such as heptyl, octyl, nonyl, decyl, and undecyl.
Examples of DILA2 amino acid compounds include R3-(C=0)-4-pyridylalanine-NH-R4 wherein the pyridylalanine is D- or L-pyridylalanine, and R3 and R4 are independently alkyl such as heptyl, octyl, nonyl, decyl, and undecyl. Examples of R3-(C=0)-pyridylalanine-NH-R4 DILA2 amino acid compounds include pharmaceutically-acceptable pyridyl salts, such as 4-[N- methylpyridyl] alanine chloride. Examples of pyridylalanine DILA2 amino acid compounds include the following structures: Compound 25
Compound 26
Compound 28
Examples of DILA2 amino acid compounds include R3-(C=0)-Lys-NH-R4 wherein R3 are independently alkyl or alkenyl.
Examples of DILA2 amino acid compounds include the following structures:
Compound 29
Compound 30
Compound 32
Examples of DELA2 amino acid compounds include R3-(C=0)-Ornithine-NH-R4 wherein and R4 are independently alkyl or alkenyl.
Examples of DILA2 amino acid compounds include the following structures:
Compound 33
Compound 34
Compound 36
Examples of DILA2 amino acid compounds include R3-(C=0)-Diaminobutryic Acid-NH-R4 wherein R3 and R4 are independently alkyl or alkenyl.
Examples of DILA2 amino acid compounds include the following structures:
Compound 37
Compound 38
Compound 42
Examples of DILA2 amino acid compounds include R3-(C=0)-His-NH-R4 wherein R3 and R4 are independently alkyl or alkenyl. Examples of His DILA2 amino acid compounds include the following structures:
Compound 43
Examples of DILA2 amino acid compounds include R3-(C=0)-Xaa-NH-R4 wherein R3 and R4 are alkyl or alkenyl. Examples of DILA2 amino acid compounds include the following structure:
Examples of DILA2 amino acid compounds include R3-(C=0)-Asp-NH-R4 wherein R3 4 are independently alkyl or alkenyl.
Examples of DELA2 amino acid compounds include the following structures:
Compound 53
Examples of DILA2 amino acid compounds include R3-(C=0)-Ser-NH-R4 wherein R3 and R4 are independently alkyl or alkenyl.
Examples of DILA2 amino acid compounds include the following structures:
Compound 54
Examples of DILA2 amino acid compounds include (ClOacyl)-Arg-NH-(Cl Oalkyl), (C 12acyl)-Arg-NH-(C 12alkyl), (C 14acyl)-Arg-NH-(C 14alkyl), (C 16acyl)-Arg-NH-(C 16alkyl), (C 18acyl)-Arg-NH-(C 18alkyl), (C 1 Oacyl)-homoArg-NH-(C 1 Oalkyl),
(C 12acyl)-homoArg-NH-(C 12alkyl), (C14acyl)-homoArg-NH-(C 14alkyl),
(C 16acyl)-homoArg-NH-(C 16alkyl), (C 18acyl)-homoArg-NH-(C 18alkyl),
(C 1 Oacyl)-norArg-NH-(C 1 Oalkyl), (C 12acyl)-nor Arg-NH-(C 12alkyl),
(C 14acyl)-nor Arg-NH-(C 14alkyl), (C 16acyl)-norArg-NH-(C 16alkyl),
(C 18acyl)-norArg-NH-(C 18alkyl), (C 1 Oacyl)-nornorArg-NH-(C 1 Oalkyl),
(CI 2acyl)-nornorArg-NH-(C 12alkyl), (C 14acyl)-nornorArg-NH-(C 14alkyl),
(C 16acyl)-nornorArg-NH-(C 16alkyl), (C 18acyl)-nornorArg-NH-(C 18alkyl),
(C 10acyl)-4-Pal-NH-(C 1 Oalkyl), (C 12acyl)-4-Pal-NH-(C 12alkyl),
(C 14acyl)-4-Pal-NH-(C 14alkyl), (C 16acyl)-4-Pal-NH-(C 16alkyl),
(C 18acyl)-4-Pal-NH-(C 18alkyl), (C 10acyl)-4-Pal(Me)-NH-(C lOalkyl),
(C12acyl)-4-Pal(Me)-NH-(C12alkyl), (C14acyl)-4-Pal(Me)-NH-(C14alkyl),
(C 16acyl)-4-Pal(Me)-NH-(C 16alkyl), and (C 18acyl)-4-Pal(Me)-NH-(C 18alkyl).
In general, the designation "C14-norArg-C14," for example, refers to
(C13alkyl)-(C=0)-norArg-NH-(C14alkyl) which is the same as
(C 14acyl)-norArg-NH-(C 14alkyl).
Examples of DILA2 amino acid compounds include (ClOacyl)-D-Arg-L-
Arg-NH-(C 1 Oalkyl), (C 12acyl)-D- Arg-L- Arg-NH-(C 12alkyl), (C 14acyl)-D-Arg-L- Arg-NH-(C 14alkyl), (C 16acyl)-D-Arg-L-Arg-NH-(C 16alkyl), (C 18acyl)-D-Arg-L- Arg-NH-(C 18alkyl), (C 1 Oacyl)-D-homoArg-L-homoArg-NH-(C 1 Oalkyl), (C 12acyl)-D- homoArg-L-homoArg-NH-(C 12alkyl), (C14acyl)-D-homoArg-L-homoArg-NH-(C14alkyl), (C 16acyl)-D-homoArg-L-homoArg-NH-(C 16alkyl), (C 18acyl)-D-homoArg-L- homoArg-NH-(C 18alkyl), (C 10acyl)-D-norArg-L-norArg-NH-(C 1 Oalkyl), (C 12acyl)-D-norArg- L-norArg-NH-(C12alkyl), (C 14acyl)-D-norArg-L-norArg-NH-(C14alkyl), (C16acyl)-D-norArg- L-norArg-NH-(C 16alkyl), (C 18acyl)-D-norArg-L-norArg-NH-(C 18alkyl), (C 10acyl)-D- nornorArg-L-nornor Arg-NH-(C 1 Oalkyl), (C 12acyl)-D-nornor Arg-L-nornor Arg-NH-(C 12alkyl),
(C 14acyl)-D-nornor Arg-L-nornor Arg-NH-(C 14alkyl), (C 16acyl)-D-nomor Arg-L- nornorArg-NH-(C 16alkyl), (C 18acyl)-D-nornorArg-L-nornorArg-NH-(C 18alkyl).
Examples of DILA2 amino acid compounds include (ClOacyl)-His-Arg-NH-(ClOalkyl), (C 12acyl)-His-Arg-NH-(C 12alkyl), (C 14acyl)-His-Arg-NH-(C 14alkyl), (C 16acyl)-His- Arg-NH-(C 16alkyl), (C 18acyl)-His-Arg-NH-(C18alkyl), (ClOacyl)-His-Arg-NH-(ClOalkyl), (C 12acyl)-His- Arg-NH-(C 12alkyl), (C 14acyl)-His-Arg-NH-(C 14alkyl), (C 16acyl)-His- Arg-NH-(C16alkyl), (C 18acyl)-His-Arg-NH-(C 18alkyl), (C 10acyl)-His-Arg-(C 1 Oalkyl), (C 12acyl)-His-Arg-NH-(C 12alkyl), (C 14acyl)-His-Arg-NH-(C 14alkyl), (C 16acyl)-His- Arg-NH-(C 16alkyl), (C 18acyl)-His-Arg-NH-(C 18alkyl), (C 10acyl)-His-Arg-NH-(C 1 Oalkyl), (C12acyl)-His-Arg-NH-(C12alkyl), (C14acyl)-His-Arg-NH-(C14alkyl), (C 16acyl)-His- Arg-NH-(C16alkyl), (C18acyl)-His-Arg-NH-(C18alkyl).
Examples of DILA2 amino acid compounds include (ClOacyl)-His-Asp-NH-(Cl Oalkyl), (C 12acyl)-His-Asp-NH-(C 12alkyl), (C 14acyl)-His-Asp-NH-(C 14alkyl), (C 16acyl)-His- Asp-NH-(C 16alkyl), (C 18acyl)-His-Asp-NH-(C 18alkyl), (C 10acyl)-His-Asp-NH-(C 1 Oalkyl), (C12acyl)-His-Asp-NH-(C12alkyl), (C14acyl)-His-Asp-NH-(C14alkyl), (C16acyl)-His- Asp-NH-(C16alkyl), (C18acyl)-His-Asp-NH-(C18alkyl), (ClOacyl)-His-Asp-(ClOalkyl), (C 12acyl)-His- Asp-NH-(C 12alkyl), (C 14acyl)-His- Asp-NH-(C 14alkyl ), (C 16acyl)-His- Asp-NH-(C 16alkyl), (C 18acyl)-His-Asp-NH-(C 18alkyl), (C 10acyl)-His-Asp-NH-(C 1 Oalkyl), (C 12acyl)-His- Asp-NH-(C 12alkyl), (C 14acyl)-His-Asp-NH-(C 14alkyl), (C 16acyl)-His- Asp-NH-(C16alkyl), (C18acyl)-His-Asp-NH-(C18alkyl).
Examples of DILA2 amino acid compounds include (ClOacyl)-Pal-Arg-NH-(Cl Oalkyl), (C 12acyl)-Pal-Arg-NH-(C 12alkyl), (C 14acyl)-Pal-Arg-NH-(C 14alkyl), (C 16acyl)-Pal- Arg-NH-(C 16alkyl), (C 18acyl)-Pal-Arg-NH-(C 18alkyl), (C 10acyl)-Pal-Arg-NH-(C 1 Oalkyl), (C 12acyl)-Pal- Arg-NH-(C 12alkyl), (C 14acyl)-Pal-Arg-NH-(C 14alkyl), (C 16acyl)-Pal- Arg-NH-(C16alkyl), (C18acyl)-Pal-Arg-NH-(C18alkyl), (ClOacyl)-Pal-Arg-(ClOalkyl), (C12acyl)-Pal-Arg-NH-(C12alkyl), (C14acyl)-Pal-Arg-NH-(C14alkyl), (C 16acyl)-Pal- Arg-NH-(C 16alkyl), (C 18acyl)-Pal-Arg-NH-(C 18alkyl), (C 10acyl)-Pal-Arg-NH-(C lOalkyl), (C 12acyl)-Pal- Arg-NH-(C 12alkyl), (C 14acyl)-Pal-Arg-NH-(C 14alkyl), (C 16acyl)-Pal- Arg-NH-(C 16alkyl), (C 18acyl)-Pal-Arg-NH-(C 18alkyl).
DILA2 amino acid compounds can be prepared as poly-mer or multi-mer species, such as dimers, trimers, or tetramers. The poly-mer or multi-mer species can be prepared from a single DILA2 amino acid compound, or from more than one species. Poly-mer or multi-mer DILA2 amino acid compounds can be prepared in some embodiments by providing a sulfhydryl group or other cross-linkable group on a side chain of the amino acid, or with linked or tethered amino
acid structures such as desmosine or citrulline. In other embodiments, a poly-mer or multi-mer DJLA2 amino acid compound can be prepared with bioconjugate linker chemistries.
Examples of DILA2 amino acid compounds include the following structures:
A DILA2 amino acid compound can be prepared as a conjugate having a peptide or polymer chain covalently attached to the amino acid side chain. The peptide or polymer chain can be attached using a reactive group of the amino acid side chain, for example, using the thiol or methylmercaptan group of cysteine or methionine, respectively, or the alcohol group of serine, or the amino group of lysine. The peptide or polymer chain can be attached using any reactive group of a substituted or modified amino acid side chain. Various linker groups such as NHS, maleimido, and bioconjugate techniques and linkers can be used.
DELA2 amino acid compounds can be prepared as constructs attached to an oligomeric or polymeric framework. For example, a DELA2 amino acid compound can be attached to polyethylene glycol, polypropylene glycol, an oligonucleotide network or lattice, a poly(amino acid), a carbohydrate, a dextran, a hydrogel, or a starch.
DBLA2 amino acid compounds can be prepared as constructs attached to a
pharmaceutical drug compound or composition, or a biologically active agent. For example, a DILA2 amino acid compound can be conjugated to a nucleic acid drug such as a regulatory or interfering RNA.
Examples of DILA2 amino acid compounds include the following structures:
where R is any amino acid side chain.
The compounds and compositions of this disclosure may incorporate solubilizing or functionalizing groups or structures including polymeric structures. See, e.g., R. L. Dunn and R. M. Ottenbrite, Polymeric Drugs and Drug Delivery Systems, ACS Symp. Ser. 469 (1991).
DILA2 amino acid compounds can be derivatized to enhance solubility such as, for example, to attach a diol, to prepare a quaternary ammonium or charged group, to attach hydroxyl or amine groups such as alcohols, polyols, or polyethers, or to attach a polyethyleneimine, a
polyethyleneglycol or a polypropyleneglycol. The molecular mass of an attached polymeric component such as a polyethyleneglycol can be any value, for example, 200, 300, 400, 500, 750, 1000, 1250, 1500, 2000, 3000, 4000, 5000, 7500, 10,000, 15,000, 20,000, 25,000, or 30,000 Da, or greater. For example, a polyethyleneglycol chain can be attached through an amino group or other reactive group of an amino acid side chain.
In general, as used herein, general chemical terms refer to all groups of a specified type, including groups having any number and type of atoms, unless otherwise specified. For example "alkenyl" refers broadly to alkyls having 2 to 22 carbon atoms, as defined below, while
(C 18: l)alkenyl refers to alkenyls having 18 carbon atoms and one double bond.
DILA2 amino acid compounds can be synthesized by methods known in the art.
Methods to prepare various organic groups and protective groups are known in the art and their use and modification is generally within the ability of one of skill in the art. See, e.g., Stanley R. Sandler and Wolf Karo, Organic Functional Group Preparations (1989); Greg T. Hermanson, Bioconjugate Techniques (1996); Leroy G. Wade, Compendium Of Organic
Synthetic Methods (1980); examples of protective groups are found in T. W. Greene and P. G. M. Wuts, Protective Groups In Organic Synthesis (3rd ed. 1991).
A pharmaceutically acceptable salt of a peptide or protein composition of this disclosure which is sufficiently basic may be an acid-addition salt with, for example, an inorganic or organic acid such as hydrochloric, hydrobromic, sulfuric, nitric, phosphoric, chlorosulfonic, trifluoroacetic, citric, maleic, acetic, propionic, oxalic, malic, maleic, malonic, fumaric, or tartaric acids, and alkane- or arenesulfonic acids such as methanesulfonic, ethanesulfonic, benzenesulfonic, chlorobenzenesulfonic, toluenesulfonic, naphthalenesulfonic,
naphthalenedisulfonic, and camphorsulfonic acids.
A pharmaceutically acceptable salt of a peptide or protein composition of this disclosure which is sufficiently acidic may be an alkali metal salt, for example, a sodium or potassium salt, or an alkaline earth metal salt, for example, a calcium or magnesium salt, or a zinc or manganese salt, or an ammonium salt or a salt with an organic base which provides a physiologically- acceptable cation, for example, a salt with methylamine, dimethylamine, trimethylamine, triethylamine, ethanolamine, diethanolamine, triethanolamine, ethylenediamine, tromethamine, N-methylglucamine, piperidine, morpholine or tris-(2-hydroxyethyl)amine, and including salts of amino acids such as arginate, and salts of organic acids such as glucuronic or galactunoric acids. See, for example, Berge et al., J. Pharm. Sci. 66: 1-19, 1977.
A salt or pharmaceutically-acceptable salt of a composition of this disclosure which contains an interfering-RNA agent and a DILA2 amino acid compound, a lipid, a peptide, or protein, among other components, may contain a salt complex of the interfering-RNA agent and the DILA2 amino acid compound, lipid, peptide, or protein. A salt complex of the interfering- RNA agent and the DILA2 amino acid compound, lipid, peptide, or protein may be formed from a pharmaceutically-acceptable salt of an interfering-RNA agent, or from a pharmaceutically- acceptable salt of the DILA2 amino acid compound, lipid, peptide, or protein.
Some compounds of this disclosure may contain both basic and acidic functionalities that may allow the compounds to be made into either a base or acid addition salt.
Some compounds, peptides and/or protein compositions of this disclosure may have one or more chiral centers and/or geometric isomeric centers (E- and Z-isomers), and it is to be
understood that the disclosure encompasses all such optical isomers, diastereoisomers, geometric isomers, and mixtures thereof.
This disclosure encompasses any and all tautomeric, solvated or unsolvated, hydrated or unhydrated forms, as well as any atom isotope forms of the compounds, peptides and/or protein compositions disclosed herein.
Lipids
In some aspects of this disclosure, one or more DILA2 amino acid compounds and one or more lipids may be employed for delivery and administration of regulatory RNA components, RNA antagonists, interfering RNA, or nucleic acids. More particularly, a composition of this disclosure may include one or more DILA2 amino acid compounds along with cationic lipids and non-cationic lipids.
Cationic lipids may be monocationic or polycationic. Some cationic lipids include neutral lipids and lipids having approximately zero net charge at a particular pH, for example, a zwitterionic lipid. Non-cationic lipids also include anionic lipids.
In some embodiments, a composition is a mixture or complex of an RNA component with a DILA2 amino acid compound and a cationic lipid. In some embodiments, a composition may be a mixture or complex of one or more regulatory or interfering RNA agents with one or more DILA2 amino acid compounds and one or more cationic lipids.
The compounds and compositions of this disclosure can be admixed with, or attached to various targeting ligands or agents to deliver an active agent to a cell, tissue, organ or region of . an organism. Examples of targeting agents include antibodies, ligands for receptors, peptides, proteins, lectins, (poly)saccharides, galactose, mannose, cyclodextrins, nucleic acids, DNA,
RNA, aptamers, and polyamino acids.
Examples of cationic lipids include N-[l-(2,3-dioleoyloxy)propyl]-N,N,N- trimethylammonium chloride (DOTMA); 1,2-bis(oleoyloxy)-3-3-(trimethylammonium)propane
(DOTAP), l,2-bis(dimyrstoyloxy)-3-3-(trimethylammonia)propane (DMTAP); 1 ,2- dimyristyloxypropyl-3-dimethylhydroxyethylammonium bromide (DMRIE);
dimethyldioctadecylammonium bromide (DDAB); 3-(N-(N',N'- dimethylaminoethane)carbamoyl)cholesterol (DC-Choi); 3P-[N',N'-diguanidinoethyl- aminoethane)carbamoyl cholesterol (BGTC); 2-(2-(3-(bis(3- aminopropyl)amino)propylamino)acetamido)-N,N-ditetradecylacetamide (RPR209120);
pharmaceutically acceptable salts thereof, and mixtures thereof.
Examples of cationic lipids include l ,2-dialkenoyl-jn-glycero-3-ethylphosphocholines (EPCs), such as l ,2-dioleoyl-in-glycero-3-ethylphosphocholine, 1,2-distearoyl-i«-glycero-3- ethylphosphocholine, 1 ,2-dipalmitoyl-in-glycero-3-ethylphosphocholine, pharmaceutically acceptable salts thereof, and mixtures thereof.
Examples of cationic lipids include 1,2-distearyloxy-N,N-dimethyl-3-aminopropane
(DSDMA), 1,2-dioleyloxy-N,Ndimethyl-3-aminopropane (DODMA), 1,2-dilinoleyloxy-N,N- dimethyl-3-aminopropane (DLinDMA), and 1,2-dilinolenyloxy-N,N-dimethyl-3-aminopropane (DLenDMA).
Examples of polycationic lipids include tetramethyltetrapalmitoyl spermine (TMTPS), tetramethyltetraoleyl spermine (TMTOS), tetramethlytetralauryl spermine (TMTLS), tetramethyltetramyristyl spermine (TMTMS), tetramethyldioleyl spermine (TMDOS), pharmaceutically acceptable salts thereof, and mixtures thereof.
Examples of polycationic lipids include 2,5-bis(3-aminopropylamino)-N-(2- (dioctadecylamino)-2-oxoethyl) pentanamide (DOGS); 2,5-bis(3-aminopropylamino)-N-(2- (di(Z)-octadeca-9-dienylamino)-2-oxoethyl) pentanamide (DOGS-9-en); 2,5-bis(3- aminopropylamino)-N-(2-(di(9Z,12Z)-octadeca-9, 12-dienylamino)-2-oxoethyl) pentanamide (DLinGS); 3-beta-(N4-(N',N8-dicarbobenzoxyspermidine)carbamoyl)cholesterol (GL-67);
(9Z,9'Z)-2-(2,5-bis(3-aminopropylamino)pentanamido)propane-1,3-diyl-dioctadec-9-enoate (DOSPER); 2,3-dioleyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium trifluoro-acetate (DOSPA); pharmaceutically acceptable salts thereof, and mixtures thereof.
Examples of cationic lipids include DS404-28 BGTC (CAS 182056-06-0), DOSPER (CAS 178532-92-8), GL-67 (179075-30-0), RPR209120 (CAS 433292-13-8), DOGS (12050-77- 7), DOGS (9-en, C18: l), DLinGS (C18:2), and DOTMA ( 104162-48-3).
Examples of cationic lipids are described in U.S. Patent Nos. 4,897,355; 5,279,833; 6,733,777; 6,376,248; 5,736,392; 5,334,761 ; 5,459,127; 2005/0064595; 5,208,036; 5,264,618; 5,279,833; 5,283,185; 5,753,613; and 5,785,992.
In some embodiments, the composition is a mixture or complex of an RNA component with a DILA2 amino acid compound and a non-cationic lipid. In some embodiments, the composition is a mixture or complex of one or more RNA components with one or more DILA2 amino acid compounds and one or more non-cationic lipids.
Non-cationic lipids include neutral, zwitterionic, and anionic lipids. Thus, a non-cationic zwitterionic lipid may contain a cationic head group.
Examples of non-cationic lipids include 1,2-Dilauroyl-sn-glycerol (DLG);
1,2-Dimyristoyl-sn-glycerol (DMG); 1 ,2-Dipalmitoyl-sn-glycerol (DPG); 1,2-Distearoyl-sn-
glycerol (DSG); l ,2-Dilauroyl-sn-glycero-3-phosphatidic acid (sodium salt; DLPA);
l ,2-Dimyristoyl-sn-glycero-3-phosphatidic acid (sodium salt; DMPA); 1,2-Dipalmitoyl-sn- glycero-3-phosphatidic acid (sodium salt; DPPA); 1 ,2-Distearoyl-sn-glycero-3-phosphatidic acid (sodium salt; DSPA); 1,2-Diarachidoyl-sn-glycero-3-phosphocholine (DAPC); 1,2-Dilauroyl-sn- glycero-3-phosphocholine (DLPC); 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC); 1 ,2- Dipalmitoyl-sn-glycero-O-ethyl-3-phosphocholine (chloride or triflate; DPePC); 1,2- Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC); 1 ,2-Distearoyl- sn-glycero-3-phosphocholine (DSPC); l ,2-Dilauroyl-sn-glycero-3-phosphoethanolamine (DLPE); 1 ,2-Dimyristoyl-sn-glycero- 3-phosphoethanolamine (DMPE); 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE); 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine (DSPE); 1,2-Dilauroyl-sn-glycero-3- phosphoglycerol (sodium salt; DLPG); 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol (sodium salt; DMPG); 1,2-Dimyristoyl-sn-glycero-3-phospho-sn-1-glycerol (ammonium salt; DMP-sn-1- G); l ,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol (sodium salt; DPPG); 1,2-Distearoyl-sn- glycero-3-phosphoglycero (sodium salt; DSPG); 1,2-Distearoyl-sn-glycero-3-phospho-sn-l - glycerol (sodium salt; DSP-sn-1-G); l ,2-Dipalmitoyl-sn-glycero-3-phospho-L-serine (sodium salt; DPPS); l-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PLinoPC); l-Palmitoyl-2- oleoyl-sn-glycero-3-phosphocholine (POPC); 1 -Palmitoyl-2-oleoyl-sn-glycero-3- phosphoglycerol (sodium salt; POPG); l-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (sodium salt; POPG); l-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (ammonium salt; POPG); l-Palmitoyl-2-4o-sn-giycero-3-phosphocholine (P-lyso-PC); l-Stearoyl-2-lyso-sn- glycero-3-phosphocholine (S-lyso-PC); and mixtures thereof.
Examples of non-cationic lipids include polymeric compounds and polymer-lipid conjugates or polymeric lipids, such as pegylated lipids having PEG regions of 300, 500, 1000, 1500, 2000, 3500, or 5000 molecular weight, including polyethyleneglycols, N-(Carbonyl- methoxypolyethyleneglycol-2000)- 1 ,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (sodium salt; DMPE-MPEG-2000); N-(Carbonyl-methoxypolyethyleneglycol-5000)- 1 ,2-dimyristoyl-sn- glycero-3-phosphoethanolamine (sodium salt; DMPE-MPEG-5000); N-(Carbonyl- methoxypolyethyleneglycol 2000)- 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (sodium salt; DPPE-MPEG-2000); N-(Carbonyl-methoxypolyethyleneglycol 5000)-1,2-dipalmitoyl-sn- glycero-3-phosphoethanolamine (sodium salt; DPPE-MPEG-5000); N-(Carbonyl- methoxypolyethyleneglycol 750)- 1 ,2-distearoyl-sn-glycero-3-phosphoethanolamine (sodium salt; DSPE-MPEG-750); N-(Carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn- glycero-3-phosphoethanolamine (sodium salt; DSPE-MPEG-2000); N-(Carbonyl- methoxypolyethyleneglycol 5000)- 1 ,2-distearoyl-sn-glycero-3-phosphoethanolamine (sodium
salt; DSPE-MPEG-5000); sodium cholesteryl sulfate (SCS); pharmaceutically acceptable salts thereof, and mixtures thereof.
Examples of non-cationic lipids include polymeric lipids such as DOPE-PEG, DLPE- PEG, DDPE-PEG DLinPE-PEG, and diacylglycerol-PEG-2000 or -5000.
Examples of non-cationic lipids include polymeric lipids such as multi-branched pegylated compounds, for example DSPE-PTE020 and DSPE-AM0530K.
Examples of non-cationic lipids include polymeric lipids such as DSPE-PG8G polyglycerine lipids.
Examples of non-cationic lipids include dioleoylphosphatidylethanolamine (DOPE), diphytanoylphosphatidylethanolamine (DPhPE), l ,2-Dioleoyl-jn-Glycero-3-Phosphocholine (DOPC), and l ,2-Diphytanoyl-jn-Glycero-3-Phosphocholine (DPhPC).
Examples of non-cationic lipids include cholesterols, sterols, and steroids such as gonanes, estranes, androstanes, pregnanes, cholanes, cholestanes, ergostanes, campestanes, poriferastanes, stigmastanes, gorgostanes, lanostanes, cycloartanes, as well as sterol or zoosterol derivatives of any of the foregoing, and their biological intermediates and precursors, which may include, for example, cholesterol, lanosterol, stigmastanol, dihydrolanosterol, zymosterol, zymostenol, desmosterol, 7-dehydrocholesterol, and mixtures and derivatives thereof.
Examples of non-cationic lipids include pegylated cholesterols, and cholestane 3-oxo(Cl- 22acyl) derivatives such as cholesteryl acetate, cholesteryl arachidonate, cholesteryl butyrate, cholesteryl hexanoate, cholesteryl caprylate, cholesteryl n-decanoate, cholesteryl dodecanoate, cholesteryl myristate, cholesteryl palmitate, cholesteryl behenate, cholesteryl stearate, cholesteryl nervonate, cholesteryl pelargonate, cholesteryl n-valerate, cholesteryl oleate, cholesteryl elaidate, cholesteryl erucate, cholesteryl heptanoate, cholesteryl linolelaidate, cholesteryl linoleate, and mixtures and derivatives thereof.
Examples of non-cationic lipids include compounds derived from plant sterols including phytosterols, beta-sitosterol, campesterol, ergosterol, brassicasterol, delta-7-stigmasterol, delta-7- avenasterol, and mixtures and derivatives thereof.
Examples of non-cationic lipids include bile acids, cholic acid, chenodeoxycholic acid, glycocholic acid, taurocholic acid, deoxycholic acid, lithocholic acid, methyl-lithocholic acid, and mixtures and derivatives thereof.
Examples of non-cationic lipids include compounds derived from steroids including glucocorticoids, Cortisol, hydrocortisone, corticosterone, A5-pregnenolone, progesterone, deoxycorticosterone, 17-OH-pregnenolone, 17-OH-progesterone, 1 1-dioxycortisol,
dehydroepiandrosterone, dehydroepiandrosterone sulfate, androstenedione, aldosterone, 18-
hydroxycorticosterone, tetrahydrocortisol, tetrahydrocortisone, cortisone, prednisone, 6a- methylpredisone, 9a-fluoro-16a-hydroxyprednisolone, 9a-fluoro- 16a-methyl prednisolone, 9a- fluorocortisol, and mixtures and derivatives thereof.
Examples of non-cationic lipids include compounds derived from steroids including adrogens, testosterone, dihydrotestosterone, androstenediol, androstenedione, androstenedione, 3a,5a-androstanediol, and mixtures and derivatives thereof.
Examples of non-cationic lipids include compounds derived from steroids including estrogens, estriols, estrones, estradiols, and mixtures and derivatives thereof.
Examples of non-cationic lipids include compounds derived from lumisterol and vitamin D compounds.
Examples of non-cationic lipids include lipids having tails ranging from C10:0 to C22:6, for example, DDPE (C10:0) (CAS 253685-27-7), DLPE (C12.0) (CAS 59752-57-7), DSPE (C18:0) (CAS 1069-79-0), DOPE (C18: l) (CAS 4004-05-1), DLinPE (C 18:2) (CAS 20707-71- 5), DLenPE (CI 8:3) (CAS 34813-40-6), DARAPE (C20:4) (CAS 5634-86-6), DDHAPE (C22:6) (CAS 123284-81-1), DPhPE (16:0[(CH3)4]) (CAS 201036-16-0).
Examples of anionic lipids include phosphatidylserine, phosphatidic acid,
phosphatidylcholine, platelet-activation factor (PAF), phosphatidylethanolamine, phosphatidyl- DL-glycerol, phosphatidylinositol, phosphatidylinositol (pi(4)p, pi(4,5)p2), cardiolipin (sodium salt), lysophosphatides, hydrogenated phospholipids, sphingoplipids, gangliosides,
phytosphingosine, sphinganines, pharmaceutically acceptable salts thereof, and mixtures thereof.
Compositions and Formulations for Administration
In some embodiments, this invention provides a method of treating a disease or disorder in a mammalian subject. A therapeutically effective amount of a composition of this invention containing an interfering RNA, a DILA2 amino acid compound, a non-cationic lipid, a polymeric lipid, and one or more delivery-enhancing components or excipients may be administered to a subject having a disease or disorder associated with expression or
overexpression of a gene that can be reduced, decreased, downregulated, or silenced by the composition.
This invention encompasses methods for treating a disease of the lung such as respiratory distress, asthma, cystic fibrosis, pulmonary fibrosis, chronic obstructive pulmonary disease, bronchitis, or emphysema, by administering to the subject a therapeutically effective amount of a composition.
This invention encompasses methods for treating a disease including cancer, bladder cancer, liver cancer, liver disease, hypercholesterolemia, an inflammatory disease, a metabolic disease, inflammation, arthritis, rheumatoid arthritis, encephalitis, bone fracture, heart disease, viral disease, hepatitis, and influenza.
Methods for making liposomes are given in, for example, G. Gregoriadis, Liposome
Technology (CRC Press 1984), and M. J. Ostro, Liposomes (Marcel Dekker 1987).
The nucleic acid component, D1LA2 amino acid compounds, and other components may be mixed together first in a suitable medium such as a cell culture medium, after which one or more lipids or compounds may be added to the mixture. Alternatively, the DILA2 amino acid compounds can be mixed together first in a suitable medium such as a cell culture medium, after which the nucleic acid component can be added.
Within certain embodiments of the invention, a dsRNA is admixed with one or more DILA2 amino acid compounds, or a combination of one or more DELA2 amino acid compounds and non-cationic lipids.
The interfering RNA agent may also be complexed with, or conjugated to a DELA2 amino acid compound or polymeric lipid, and admixed with one or more non-cationic lipids, or a combination of one or more non-cationic and cationic lipids.
An interfering RNA agent and a DILA2 amino acid compound may be mixed together first, followed by the addition of one or more non-cationic lipids, or a combination of non- cationic and cationic lipids added in a suitable medium such as a cell culture medium.
Alternatively, the DILA2 amino acid compounds and lipid components may be mixed first, followed by the addition of the RNA agent in a suitable medium.
In some embodiments, this disclosure includes micellar dispersion compositions containing a drug or active agent admixed or complexed with one or more DILA2 amino acid compounds and a dispersant to form a composition that provides intracellular delivery of the drug or active agent.
In certain embodiments, a dispersion composition of this disclosure may contain one or more drugs or active agents, one or more DILA2 amino acid compounds, and one or more dispersants. In some variations, a delivery composition may contain a drug or active agent, a dispersant, a DILA2 amino acid compound, and an optional polymeric lipid. The dispersion compositions of this disclosure can form stable particles which may incorporate the drug or active agent.
In some aspects, a dispersion composition of this disclosure may contain stable nucleic acid dispersion particles having diameters from about 5 nm to about 400 nm. In some
embodiments, the particles may have a uniform diameter of from about 10 nm to about 300 nm. In some embodiments, the particles may have a uniform diameter of from about 50 nm to about 150 nm.
A micellar dispersion can be used to formulate and improve the bioavailability of a drug or active agent, including RNAi therapeutics. A micellar dispersion can provide dispersion droplets or nanoparticles having a hydrophobic oil-like core. The dispersion nanoparticles can be suspended in a continuous aqueous phase. A dispersion structure can avoid some disadvantages inherent in using a liposomal structure for delivery of active agents, and can provide advantages in delivery because of the lipophilic core.
This disclosure provides a range of micellar dispersion compositions containing DILA2 amino acid compounds or lipids and dispersants for drugs or medicaments, and for delivery and administration of RNA agents.
Examples of dispersants include synthetic compounds including polyoxyglycerides such as polyglycolated capryl glycerides, ethoxy diglycol, pegylated fatty glycerides, diethylene glycol monoethyl ethers, and mixtures thereof. Examples of dispersants include LABRAFIL, LABRASOL, ARLATONE, TRANSCUTOL, and mixtures thereof. Examples of dispersants include synthetic compounds such as alkylphospho-N-methylethanolarnines and
alkoylsarcosines. Examples of dispersants include FOS-MEA and CRODASINIC.
In some embodiments, a delivery composition of this disclosure may contain a drug or active agent, one or more oils, one or more DILA2 amino acid compounds, and emulsifier and stabilizer lipids. In some variations, a delivery composition may contain a drug or active agent, an oil, a lipid emulsifier, a DILA2 amino acid compound, a non-cationic lipid, and a polymeric lipid.
The compositions of this disclosure can form stable particles which may incorporate a drug or active agent. In some aspects, compositions of this disclosure contain stable drug or active agent emulsion particles having diameters from about 5 nm to about 400 nm. In some embodiments, the particles may have a uniform diameter of from about 10 nm to about 300 nm. In some embodiments, the particles may have a uniform diameter of from about 50 nm to about 150 nm.
In some embodiments, a drug or active agent may be admixed or complexed with an oil, an emulsifier, a DILA2 amino acid compound, and a polymeric stabilizing lipid, to form a composition that enhances intracellular delivery of the drug or active agent.
An oil-in-water emulsion can be used to formulate and improve the bioavailability of a drug or active agent, including RNAi therapeutics.
An oil-in-water emulsion can provide emulsion droplets or nanoparticles having a DELA2 amino acid compound or lipid layer surrounding a hydrophobic oil core. The emulsion droplets or nanoparticles can be suspended in a continuous aqueous phase. An emulsion structure can avoid some disadvantages inherent in using a liposomal structure for delivery of active agents, and can provide advantages in delivery because of the lipophilic core.
A range of novel emulsion compositions are provided in this disclosure including novel compositions and uses of oils, emulsifiers, DILA2 amino acid compounds and lipid components with interfering-RNA agents.
Examples of oils include synthetic oils, fatty acid esters of propylene glycols, ethers of ethylene glycols, glyceryl oils, cholesteryl oils, vegetable oils, nut oils, essential oils, mineral oil, lipid-soluble compounds such as tocopherols and Vitamin E, and mixtures thereof. Examples of oils include synthetic oils such as CAPRYOL 90 (propylene glycol monoester), CAPRYOL PGMC (propylene glycol monoester), LABRAFAC PC (propylene glycol monoester),
LABRAFAC PG (propylene glycol diester), LAUROGLYCOL 90 (propylene glycol monoester), LAUROGLYCOL FCC (propylene glycol monoester), PLUROL OLEIQUE CC 497 (propylene glycol monoester), LABRAFAC LIPOPHILE WL 1349 (triglyceride), PECEOL (glyceryl monoester), MAISINE 35-1 (glyceryl monoester), and mixtures thereof.
In exemplary embodiments, this disclosure includes compositions containing a nucleic acid molecule, such as a double-stranded RNA (dsRNA), a short interfering RNA (siRNA), or a short hairpin RNA (shRNA), admixed or complexed with a DELA2 amino acid compound, and a polymeric lipid to form a composition that enhances intracellular delivery of the nucleic acid molecule. In some embodiments, a delivery composition of this disclosure may contain a dsRNA and one, two, or more DILA2 amino acid compounds, which may be cationic or non- cationic. In some variations, a delivery composition may contain a dsRNA, DILA2 amino acid compounds, and one or more polymeric lipids. In some embodiments, a delivery composition may contain a dsRNA, one or more DILA2 amino acid compounds, one or more lipids, and one or more polymeric lipids. The compositions of this disclosure can form stable particles which may incorporate a dsRNA as an interfering RNA agent. Compositions and formulations of this disclosure may include further delivery-enhancing components or excipients.
In some embodiments, compositions of this disclosure contain stable nucleic acid containing nanoparticles (e.g., RNA-containing particles) having diameters from about 5 nm to about 400 nm, In some embodiments, the nanoparticles may have a uniform diameter of from about 10 nm to about 300 nm. In some embodiments, the nanoparticles may have a uniform diameter of from about 50 nm to about 150 nm. In some embodiments, the nanoparticles have
diameters from about 50 nm to about 250 nm. In some embodiments, the nanoparticles have diameters from about 60 nm to about 150 nm. In some embodiments, the nanoparticles have diameters from about 70 nm to about 120 nm ( or 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 , 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 1 10, 1 11, 112, 1 13, 1 14, 1 15, 1 16, 1 17, 1 18, 1 19, or 120 nm).
In some embodiments, compositions of this disclosure contain nucleic acid containing nanoparticles (e.g., RNA-containing nanoparticles) having an N/P ratio of from about 0.1 to about 20. In some embodiments, the nanoparticles have an N/P ratio of from about 1 to about 10. In some embodiments, the nanoparticles have an N P ratio of from about 1.5 to about 5 (or 1.5, 1.6, 1.7, 1.8, 1.9, 2, 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 3, 3.1, 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4, 4.1, 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9, or 5).
In some embodiments, compositions of this disclosure contain nucleic acid containing nanoparticles (e.g., RNA-containing nanoparticles) having a charge ratio of from about 0.1 to about 3. In some embodiments, the nanoparticles have a charge ratio of from about 0.5 to about 1.5. In some embodiments, the nanoparticles have a charge ratio of from about 0.9 to about 1.1 (or 0.9. 0.95, 1 , 1.05, or 1.1).
In some embodiments, compositions of this disclosure contain nucleic acid containing nanoparticles (e.g., RNA-containing nanoparticles) having a C/N ratio of from about 0.1 to about 5. In some embodiments, the nanoparticles have a C/N ratio of from about 0.5 to about 2. In some embodiments, the nanoparticles have a C/N ratio of from about 1 to about 1.2 (or 1, 1.05, 1.1 , 1.15, or 1.2).
In some embodiments, compositions of this disclosure contain nucleic acid containing nanoparticles (e.g., RNA-containing nanoparticles) having a delivery efficiency ratio (DER) of from about 1 to about 50. In some embodiments, the nanoparticles have a DER of from about 2 to about 20. In some embodiments, the nanoparticles have a DER of from about 3 to about 15 (or 3, 4, 5, 6, 7, 8, 9, 10, 1 1 , 12, 13, 14, or 15).
In some embodiments, compositions of this disclosure contain nucleic acid containing nanoparticles (e.g., RNA-containing nanoparticles) having a carrier charge ratio of from about 0.5 to about 5. In some embodiments, the nanoparticles have a carrier charge ratio of from about 0.8 to about 3. In some embodiments, the nanoparticles have a carrier charge ratio of from about 1.5 to about 2.5 (or 1.5, 1.6, 1.7, 1.8, 1.9, 2, 2.1 , 2.2, 2.3, 2.4 or 2.5).
In some embodiments, compositions of this disclosure contain nucleic acid containing nanoparticles (e.g., RNA-containing nanoparticles) having a delta charge ratio (Δ charge ratio) of from about 0.1 to about 10. In some embodiments, the nanoparticles have a Δ charge ratio of
from about 0.5 to about 4. In some embodiments, the nanoparticles have a Δ charge ratio of from about 0.8 to about 2 (or 0.8, 0.9, 1, 1.1, 1.2, 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, or 2).
In some embodiments, compositions of this disclosure contain nucleic acid containing nanoparticles (e.g., RNA-containing nanoparticles) having a zeta potential of from about -40 mV to about 0 mV, at a pH of about 7.4. In some embodiments, the nanoparticles have a zeta potential of from about -25 mV to about 0 mV, at a pH of about 7.4. In some embodiments, the nanoparticles have a zeta potential of from about - 15 mV to about -5 mV, at a pH of about 7.4. In some embodiments, the nanoparticles have a zeta potential of from about 0 mV to about 40 mV, at a pH of about 4. In some embodiments, the nanoparticles have a zeta potential of from about 0 mV to about 25 mV, at a pH of about 4. In some embodiments, the nanoparticles have a zeta potential of from about 0 mV to about 15 mV, at a pH of about 4.
Within exemplary compositions of this disclosure, a double-stranded RNA may be admixed or complexed with DILA2 amino acid compounds to form a composition that enhances intracellular delivery of the dsRNA as compared to contacting target cells with naked dsRNA.
In some embodiments, a composition of this disclosure may contain one or more DELA2 amino acid compounds which are from about 0.5% to about 70% (mol%) of the total amount of DILA2 amino acid compounds and lipids, if any, and delivery-enhancing components, including any polymeric component, but not including the RNA component. In some embodiments, a composition of this disclosure may contain one or more DILA2 amino acid compounds from about 10% to about 55%. In some embodiments, a composition of this disclosure may contain one or more DILA2 amino acid compounds from about 15% to about 35%.
In certain embodiments, a composition of this disclosure may contain one or more non-cationic lipids, where the non-cationic lipids are from about 2% to about 95% (mol%) of the total amount of DILA2 amino acid compounds and lipids, if any, and delivery-enhancing components, including any polymeric component, but not including the RNA component. In some embodiments, a composition of this disclosure may contain one or more non-cationic lipids from about 20% to about 75%, or from about 45% to about 75%, or from about 45% to about 55%. In some embodiments, a composition of this disclosure may contain one or more non-cationic lipids from about 10% to about 50%.
In some embodiments, a composition of this disclosure may contain one or more polymeric lipids, where the polymeric lipids are from about 0.2% to about 20% (mpl%) of the total amount of DILA2 amino acid compounds and lipids, if any, and delivery-enhancing components, including any polymeric component, but not including the RNA component. In some embodiments, a composition of this disclosure may contain one or more polymeric lipids
from about 0.5% to about 10%. In some embodiments, a composition of this disclosure may contain one or more polymeric lipids from about 1 % to about 5% of the composition.
Some methods for evaluating encapsulation, sizing, and general preparation of nanoparticles are given, for example, in WO2001005374, U.S. Pat. Publ. Nos. 20040142025 and 20070252295, and U.S. Pat. No. 6,843,942.
Methods and processes for making a nanoparticle composition of an active agent, for example nuclei acid, are provided in patent application PCT US2009/60930, the contents of which is incorporated by reference in its entirety.
In certain embodiments, aqueous suspensions contain dsRNA of this disclosure in admixture with suitable excipients, such as suspending agents or dispersing or wetting agents. Exemplary suspending agents include sodium carboxymethylcellulose, methylcellulose, hydropropyl-methylcellulose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum acacia. Representative dispersing or wetting agents include naturally-occurring phosphatides (e.g., lecithin), condensation products of an alkylene oxide with fatty acids (e.g.,
polyoxyethylene stearate), condensation products of ethylene oxide with long chain aliphatic alcohols (e.g., heptadecaethyleneoxycetanol), condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol (e.g., polyoxyethylene sorbitol monooleate), or condensation products of ethylene oxide with partial esters derived from fatty acids and hexitol anhydrides (e.g., polyethylene sorbitan monooleate). In certain embodiments, the aqueous suspensions can optionally contain one or more preservatives (e.g., ethyl or n-propyl-p- hydroxybenzoate), one or more coloring agents, one or more flavoring agents, or one or more sweetening agents (e.g., sucrose, saccharin). In additional embodiments, dispersible powders and granules suitable for preparation of an aqueous suspension by the addition of water provide dsRNA of this disclosure in admixture with a dispersing or wetting agent, suspending agent and optionally one or more preservative, coloring agent, flavoring agent, or sweetening agent.
The present disclosure includes dsRNA compositions prepared for storage or administration that include a pharmaceutically effective amount of a desired compound in a pharmaceutically acceptable carrier or diluent. Acceptable carriers or diluents for therapeutic use are well known in the pharmaceutical art, and are described, for example, in Remington's Pharmaceutical Sciences, Mack Publishing Co., A.R. Gennaro edit., 1985, hereby incorporated by reference herein. In certain embodiments, pharmaceutical compositions of this disclosure can optionally include preservatives, antioxidants, stabilizers, dyes, flavoring agents, or any combination thereof. Exemplary preservatives include sodium benzoate, sorbic acid, chlorobutanol, and esters of p-hydroxybenzoic acid.
The dsRNA compositions of the instant disclosure can be effectively employed as pharmaceutically-acceptable formulations. Pharmaceutically-acceptable formulations prevent, alter the occurrence or severity of, or treat (alleviate one or more symptom(s) to a detectable or measurable extent) of a disease state or other adverse condition in a subject. A pharmaceutically acceptable formulation includes salts of the above compounds, e.g., acid addition salts, such as salts of hydrochloric acid, hydrobromic acid, acetic acid, or benzene sulfonic acid. A pharmaceutical composition or formulation refers to a composition or formulation in a form suitable for administration into a cell, or a subject such as a human (e.g., systemic
administration). The formulations of the present disclosure, having an amount of dsRNA sufficient to treat or prevent a disorder associated with HRAS gene expression are, for example, suitable for topical (e.g., creams, ointments, skin patches, eye drops, ear drops) application or administration. Other routes of administration include oral, parenteral, sublingual, bladder washout, vaginal, rectal, enteric, suppository, nasal, and inhalation. The term parenteral, as used herein, includes subcutaneous, intravenous, intramuscular, intraarterial, intraabdominal, intraperitoneal, intraarticular, intraocular or retrobulbar, intraaural, intrathecal, intracavitary, intracelial, intraspinal, intrapulmonary or transpulmonary, intrasynovial, and intraurethral injection or infusion techniques. The pharmaceutical compositions of the present disclosure are formulated to allow the dsRNA contained therein to be bioavailable upon administration to a subject.
In further embodiments, dsRNA of this disclosure can be formulated as oily suspensions or emulsions (e.g., oil-in- water) by suspending dsRNA in, for example, a vegetable oil (e.g., arachis oil, olive oil, sesame oil or coconut oil) or a mineral oil (e.g., liquid paraffin). Suitable emulsifying agents can be naturally-occurring gums (e.g. , gum acacia or gum tragacanth), naturally-occurring phosphatides (e.g., soy bean, lecithin, esters or partial esters derived from fatty acids and hexitol), anhydrides (e.g., sorbitan monooleate), or condensation products of partial esters with ethylene oxide (e.g., polyoxyethylene sorbitan monooleate). In certain embodiments, the oily suspensions or emulsions can optionally contain a thickening agent, such as beeswax, hard paraffin or cetyl alcohol. In related embodiments, sweetening agents and flavoring agents can optionally be added to provide palatable oral preparations. In yet other embodiments, these compositions can be preserved by optionally adding an anti-oxidant, such as ascorbic acid.
In further embodiments, dsRNA of this disclosure can be formulated as syrups and elixirs with sweetening agents (e.g., glycerol, propylene glycol, sorbitol, glucose or sucrose). Such formulations can also contain a demulcent, preservative, flavoring, coloring agent, or any
combination thereof. In other embodiments, pharmaceutical compositions comprising dsRNA of this disclosure can be in the form of a sterile, injectable aqueous or oleaginous suspension. The sterile injectable preparation can also be a sterile, injectable solution or suspension in a non-toxic parenterally acceptable diluent or solvent (e.g., as a solution in 1,3-butanediol). Among the exemplary acceptable vehicles and solvents useful in the compositions of this disclosure is water, Ringer's solution, or isotonic sodium chloride solution. In addition, sterile, fixed oils may be employed as a solvent or suspending medium for the dsRNA of this disclosure. For this purpose, any bland fixed oil can be employed including synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid find use in the preparation of parenteral formulations.
Within certain embodiments of this disclosure, pharmaceutical compositions and methods are provided that feature the presence or administration of one or more dsRNA or analogs thereof of this disclosure, combined, complexed, or conjugated with a polypeptide, optionally formulated with a pharmaceutically-acceptable carrier, such as a diluent, stabilizer, buffer, or the like. The negatively charged dsRNA molecules of this disclosure may be administered to a patient by any standard means, with or without stabilizers, buffers, or the like, to form a composition suitable for treatment. When it is desired to use a liposome delivery mechanism, standard protocols for formation of liposomes can be followed. The compositions of the present disclosure may also be formulated and used as a tablet, capsule or elixir for oral administration, suppository for rectal administration, sterile solution, or suspension for injectable administration, either with or without other compounds known in the art. Thus, dsRNAs of the present disclosure may be administered in any form, such as nasally, transdermally, parenterally, or by local injection.
In another embodiment, a dsRNA of this disclosure can include a conjugate member on one or more of the terminal nucleotides of a dsRNA. The conjugate member can be, for example, a lipophile, a terpene, a protein binding agent, a vitamin, a carbohydrate, or a peptide. For example, the conjugate member can be naproxen, nitroindole (or another conjugate that contributes to stacking interactions), folate, ibuprofen, or a C5 pyrimidine linker. In other embodiments, the conjugate member is a glyceride lipid conjugate (e.g., a dialkyl glyceride derivatives), vitamin E conjugates, or thio-cholesterols. Additional conjugate members include peptides that function, when conjugated to a modified dsRNA of this disclosure, to facilitate delivery of the dsRNA into a target cell, or otherwise enhance delivery, stability, or activity of the dsRNA when contacted with a biological sample (e.g. , a target cell expressing HRAS).
Exemplary peptide conjugate members for use within these aspects of this disclosure, include peptides PN27, PN28, PN29, PN58, PN61, PN73, PN158, PN159, PN173, PN182, PN183,
PN202, PN204, PN250, PN361, PN365, PN404, PN453, PN509, and PN963, described, for example, in U.S. Patent Application Publication Nos. 2006/0040882 and 2006/0014289, and U.S. Provisional Patent Application Nos. 60/822,896 and 60/939,578; and PCT Application PCT/US2007/075744, which are all incorporated herein by reference. In certain embodiments, when peptide conjugate partners are used to enhance delivery of dsRNA of this disclosure, the resulting dsRNA formulations and methods will often exhibit further reduction of an interferon response in target cells as compared to dsRNAs delivered in combination with alternate delivery vehicles, such as lipid delivery vehicles (e.g. , Lipofectamine™).
In still another embodiment, a dsRNA or analog thereof of this disclosure may be conjugated to the polypeptide and admixed with one or more non-cationic lipids or a
combination of a non-cationic lipid and a cationic lipid to form a composition that enhances intracellular delivery of the dsRNA as compared to delivery resulting from contacting the target cells with a naked dsRNA. In more detailed aspects of this disclosure, the mixture, complex or conjugate comprising a dsRNA and a polypeptide can be optionally combined with (e.g., admixed or complexed with) a cationic lipid, such as Lipofectamine™. To produce these compositions comprised of a polypeptide, dsRNA and a cationic lipid, the dsRNA and peptide may be mixed together first in a suitable medium such as a cell culture medium, after which the cationic lipid is added to the mixture to form a dsRNA/delivery peptide/cationic lipid
composition. Optionally, the peptide and cationic lipid can be mixed together first in a suitable medium such as a cell culture medium, followed by the addition of the dsRNA to form the dsRNA/delivery peptide/cationic lipid composition.
This disclosure also features the use of dsRNA compositions comprising
surface-modified liposomes containing, for example, poly(ethylene glycol) lipids (PEG- modified, or long-circulating liposomes or stealth liposomes) (Lasic et al., Chem. Rev. 95:2601, 1995; Ishiwata et al, Chem. Pharm. Bull. 43: 1005, 1995; Lasic et al., Science 267: 1275, 1995; Oku et al, Biochim. Biophys. Acta 1238.S6, 1995; Liu et al., J. Biol. Chem. 42:24864, 1995; PCT Publication Nos. WO 96/10391 ; WO 96/10390; WO 96/10392).
In another embodiment, compositions are provided for targeting dsRNA molecules of this disclosure to specific cell types, such as hepatocytes. For example, dsRNA can be complexed or conjugated glycoproteins or synthetic glycoconjugates glycoproteins or synthetic glycoconjugates having branched galactose (e.g., asialoorosomucoid), N-acetyl-D-galactosamine, or mannose (see, e.g., Wu and Wu, J. Biol. Chem. 262:4429, 1987; Baenziger and Fiete, Cell 22: - 61 1 , 1980; Connolly et al., J. Biol. Chem. 257:939, 1982; Lee and Lee, Glycoconjugate J. 4:317,
1987; Ponpipom et ai, J. Med. Chem. 24: 1388, 1981) for a targeted delivery to, for example, the liver.
A pharmaceutically effective dose is that dose required to prevent, inhibit the occurrence of, or treat (alleviate a symptom to some extent, preferably all of the symptoms) a disease state. The pharmaceutically effective dose depends on the type of disease, the composition used, the route of administration, the type of subject being treated, the physical characteristics of the specific subject under consideration for treatment, concurrent medication, and other factors that those skilled in the medical arts will recognize. For example, an amount between 0.1 mg/kg and 100 mg/kg body weight/day of active ingredients may be administered depending on the potency of a dsRNA of this disclosure.
A specific dose level for any particular patient depends upon a variety of factors including the activity of the specific compound employed, age, body weight, general health, sex, diet, time of administration, route of administration, rate of excretion, drug combination, and the severity of the particular disease undergoing therapy. Following administration of dsRNA compositions as disclosed herein, test subjects will exhibit about a 10% up to about a 99% reduction in one or more symptoms associated with the disease or disorder being treated, as compared to placebo-treated or other suitable control subjects.
Dosage levels in the order of about 0.1 mg to about 140 mg per kilogram of body weight per day can be useful in the treatment of the above-indicated conditions (about 0.5 mg to about 7 g per patient per day). The amount of active ingredient that can be combined with the carrier materials to produce a single dosage form varies depending upon the host treated and the particular mode of administration. Dosage unit forms generally contain between from about 1 mg to about 500 mg of an active ingredient.
A dosage form of a dsRNA or composition thereof of this disclosure can be liquid, an emulsion, or a micelle, or in the form of an aerosol or droplets. A dosage form of a dsRNA or composition thereof of this disclosure can be solid, which can be reconstituted in a liquid prior to administration. The solid can be administered as a powder. The solid can be in the form of a capsule, tablet, or gel. In addition to in vivo gene inhibition, a skilled artisan will appreciate that the dsRNA and analogs thereof of the present disclosure are useful in a wide variety of in vitro applications, such as scientific and commercial research {e.g., elucidation of physiological pathways, drug discovery and development), and medical and veterinary diagnostics.
Nucleic acid molecules and polypeptides can be administered to cells by a variety of methods known to those of skill in the art, including administration within formulations that comprise a dsRNA alone, a dsRNA and a polypeptide complex / conjugate alone, or that further
comprise one or more additional components, such as a pharmaceutically acceptable carrier, diluent, excipient, adjuvant, emulsifier, stabilizer, preservative, or the like. Other exemplary substances used to approximate physiological conditions include pH adjusting and buffering agents, tonicity adjusting agents, and wetting agents, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, sorbitan monolaurate, triefhanolamine oleate, and mixtures thereof. For solid compositions, conventional nontoxic pharmaceutically acceptable carriers can be used which include, for example, pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, talcum, cellulose, glucose, sucrose, magnesium carbonate, and the like.
In certain embodiments, the dsRNA and compositions thereof can be encapsulated in liposomes, administered by iontophoresis, or incorporated into other vehicles, such as hydrogels, cyclodextrins, biodegradable nanocapsules, bioadhesive microspheres, or proteinaceous vectors (see, e.g. , PCT Publication No. WO 00/53722). In certain embodiments of this disclosure, the dsRNA may be administered in a time release formulation, for example, in a composition that includes a slow release polymer. The dsRNA can be prepared with carriers that will protect against rapid release, for example, a controlled release vehicle such as a polymer,
microencapsulated delivery system, or bioadhesive gel. Prolonged delivery of the dsRNA, in various compositions of this disclosure can be brought about by including in the composition agents that delay absorption, for example, aluminum monosterate hydrogels and gelatin.
Alternatively, a dsRNA composition of this disclosure can be locally delivered by direct injection or by use of, for example, an infusion pump. Direct injection of dsRNAs of this disclosure, whether subcutaneous, intramuscular, or intradermal, can be done by using standard needle and syringe methodologies or by needle-free technologies, such as those described in Corny et al, Clin. Cancer Res. 5:2330, 1999 and PCT Publication No. WO 99/31262.
The dsRNA of this disclosure and compositions thereof may be administered to subjects by a variety of mucosal administration modes, including oral, rectal, vaginal, intranasal, intrapulmonary, or transdermal delivery, or by topical delivery to the eyes, ears, skin, or other mucosal surfaces. In one embodiment, the mucosal tissue layer includes an epithelial cell layer, which can be pulmonary, tracheal, bronchial, alveolar, nasal, buccal, epidermal, or
gastrointestinal. Compositions of this disclosure can be administered using conventional actuators, such as mechanical spray devices, as well as pressurized, electrically activated, or other types of actuators. The dsRNAs can also be administered in the form of suppositories, e.g., for rectal administration. For example, these compositions can be mixed with an excipient that
is solid at room temperature but liquid at the rectal temperature so that the dsRNA is released. Such materials include, for example, cocoa butter and polyethylene glycols.
Further methods for delivery of nucleic acid molecules, such as the dsRNAs of this disclosure, are described, for example, in Boado et al, J. Pharm. Sci. 87: 1308, 1998; Tyler et al., FEBS Lett. 421:2m, 1999; Pardridge et al, Proc. Nat'l Acad. Sci. USA 92:5592, 1995; Boado, Adv. Drug Delivery Rev. 75:73, 1995; Aldrian-Herrada et al., Nucleic Acids Res. 26:4910, 1998; Tyler et al., Proc. Nat'l Acad. Sci. USA 96:7053-7058, 1999; Akhtar et al., Trends Cell Bio. 2: 139, 1992; "Delivery Strategies for Antisense Oligonucleotide Therapeutics," ed. Akhtar, 1995, Maurer et al., Mol. Membr. Biol. 16: 129, 1999; Hofland and Huang, Handb. Exp.
Pharmacol 137: 165, 1999; and Lee et al, ACS Symp. Ser. 752: 184, 2000; PCT Publication No. WO 94/02595.
Therapeutics and Methods of Use
As set forth herein, dsRNA of the instant disclosure are designed to target a HRAS gene (including one or more mRNA splice variant thereof) that is expressed at an elevated level or continues to be expressed when it should not, and is a causal or contributing factor associated with, for example, leukemia, cutaneous melanoma, adenocarcinoma, squamous cell carcinoma, Philadelphia chromosome-negative myeloproliferative disorder, myelodysplastic syndrome, transitional cell carcinoma, ovarian cancer, brain tumors, breast cancer, bladder cancer, lung cancer, kidney tumors, urinary tract tumors, pancreatic carcinoma, and colorectal adenoma; as well as one or more angiogenic diseases or disorders, hepatocellular carcinoma (HCC), NSCLC (lung nonsmall cell lung cancer), melanoma, colon cancer, prostate cancer, and glioblastoma, or adverse condition. In this context, a dsRNA or analog thereof of this disclosure will effectively downregulate expression of a HRAS gene to levels that prevent, alleviate, or reduce the severity or recurrence of one or more associated disease symptoms. Alternatively, for various distinct disease models in which expression of a HRAS gene is not necessarily elevated as a
consequence or sequel of disease or other adverse condition, down regulation of a HRAS gene will nonetheless result in a therapeutic result by lowering gene expression (i.e. , to reduce levels of a selected mRNA or protein product of a HRAS gene). Furthermore, dsRNAs of this disclosure may be targeted to lower expression of HRAS, which can result in upregulation of a "downstream" gene whose expression is negatively regulated, directly or indirectly, by a HRAS protein. The dsRNA molecules of the instant disclosure comprise useful reagents and can be used in methods for a variety of therapeutic, diagnostic, target validation, genomic discovery, genetic engineering, and pharmacogenomic applications.
In accordance with this disclosure, dsRNA molecules (optionally substituted or modified or conjugated), compositions thereof, and methods for inhibiting expression of a HRAS gene in a cell or organism are provided. In certain embodiments, this disclosure provides methods and dsRNA compositions for treating a subject, including a human cell, tissue or individual, having a disease or at risk of developing a disease caused by or associated with the expression of a HRAS gene. In one embodiment, the method includes administering a dsRNA of this disclosure or a pharmaceutical composition containing the dsRNA to a cell or an organism, such as a mammal, such that expression of the target gene is silenced. Subjects (e.g., mammalian, human) amendable for treatment using the dsRNA molecules (optionally substituted or modified or conjugated), compositions thereof, and methods of the present disclosure include those suffering from one or more disease or condition mediated, at least in part, by overexpression or inappropriate expression of a HRAS gene, or which are amenable to treatment by reducing expression of a HRAS protein, including coronary artery disease (i.e., coronary heart disease, ischaemic heart disease), atherosclerosis, diabetes mellitus, dyslipidemia (e.g., hyperlipidemia), peripheral vascular and ischemic cerebrovascular disease, and risk of ischemic stroke (cerebral thrombosis and cerebral embolisms) and hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage). Within exemplary embodiments, the compositions and methods of this disclosure are also useful as therapeutic tools to regulate expression of HRAS to treat or prevent symptoms of, for example, the conditions listed herein.
Within additional aspects of this disclosure, combination formulations and methods are provided comprising an effective amount of one or more dsRNA of the present disclosure in combination with one or more secondary or adjunctive active agents that are formulated together or administered coordinately with the dsRNA of this disclosure to control a HRAS-associated disease or condition as described herein. Useful adjunctive therapeutic agents in these combinatorial formulations and coordinate treatment methods include, for example, dsRNAs that target and decrease the expression of other genes whose aberrant expression is related to a disease or condition described herein (e.g., bladder cancer and/liver cancer), enzymatic nucleic acid molecules, allosteric nucleic acid molecules, antisense, decoy, or aptamer nucleic acid molecules, antibodies such as monoclonal antibodies, small molecules and other organic or inorganic compounds including metals, salts and ions, and other drugs and active agents indicated for treating a HRAS-associated disease or condition, including chemotherapeutic agents used to treat cancer, steroids, non-steroidal anti-inflammatory drugs (NSAIDs), tyrosine kinase inhibitors, or the like.
Exemplary chemotherapeutic agents include alkylating agents (e.g. , cisplatin, oxaliplatin, carboplatin, busulfan, nitrosoureas, nitrogen mustards, uramustine, temozolomide),
antimetabolites (e.g. , aminopterin, methotrexate, mercaptopurine, fluorouracil, cytarabine), taxanes (e.g., paclitaxel, docetaxel), anthracyclines (e.g. , doxorubicin, daunorubicin, epirubicin, idaruicin, mitoxantrone, valrubicin), bleomycin, mytomycin, actinomycin, hydroxyurea, topoisomerase inhibitors (e.g., camptothecin, topotecan, irinotecan, etoposide, teniposide), monoclonal antibodies (e.g., alemtuzumab, bevacizumab, cetuximab, gemtuzumab,
panitumumab, rituximab, tositumomab, trastuzumab), vinca alkaloids (e.g., vincristine, vinblastine, vindesine, vinorelbine), cyclophosphamide, prednisone, leucovorin, oxaliplatin.
Some adjunctive therapies may be directed at targets that interact or associate with
HPvAS or affect specific HRAS biological activities. Adjunctive therapies include statins (e.g., rosuvastatin, lovastatin, atorvastatin, cerivastatin, fluvastatin, mevastatin, pitavastatin, pravastatin, simvastatin), bile acid-binding resins, stanol and sterol esters from plants, and inhibitors of cholesterol absorption, fibrates (e.g., fenofibrate, bezafibrate, ciprofibrate, clofibrate, gemfibrozil), niacin, fish-oils, ezetimibe, amlodipine, other lipid-altering agents, additional small molecules, rationally designed peptides, and antibodies or fragments thereof.
Exemplary genes that may be targeted via the RNAi pathway by way of a dsRNA and used in combination with a dsRNA of this disclosure that controls expression of a HRAS gene include, but are not limited to, epidermal growth factor receptor (EGFR; see
PCT US2008/055360, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the EGFR gene), fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor; FLT1 or VEGFR-1 ; see PCT US2008/055370, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the VEGFR-1 gene), vascular endothelial growth factor A (VEGF-A; see PCT/US2008/055383, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the VEGF-A gene), v-akt murine thymoma viral oncogene homolog 1 (AKT1 ; see PCT/US2008/055339, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the AKT1 gene), breakpoint cluster region (BCR)-abelson murine leukemia viral oncogene homology (ABL) or BCR-ABL (see PCT US2008/055378, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the BCR-ABL gene), hypoxia-inducible factor 1 , alpha subunit (HIF1A; see PCT US2008/055385, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the HIFl A gene), FK506 binding protein 12-
rapamycin associated protein 1 (FRAPl ; see PCT/US2008/055365, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the FRAPl gene), RAFl (see PCT/US2008/055366, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the RAFl gene, protein kinase N3 (PKN3; see PCT/US2008/055386, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the PKN3 gene), and platelet-derived growth factor receptor, alpha polypeptide (PDGFRA; see PCT/US2008/055357, specifically the claims and sequence listing for guidance with respect to selecting particular dsRNAs that down-regulate the PDGFRA gene), in which the above cited PCT patent application are incorporated herein by reference.
To practice the coordinate administration methods of this disclosure, a dsRNA is administered, simultaneously or sequentially, in a coordinated treatment protocol with one or more of the secondary or adjunctive therapeutic agents contemplated herein. The coordinate administration may be done in any order, and there may be a time period while only one or both (or all) active therapeutic agents, individually or collectively, exert their biological activities. A distinguishing aspect of all such coordinate treatment methods is that the dsRNA present in a composition elicits some favorable clinical response, which may or may not be in conjunction with a secondary clinical response provided by the secondary therapeutic agent. For example, the coordinate administration of the dsRNA with a secondary therapeutic agent as contemplated herein can yield an enhanced (synergistic) therapeutic response beyond the therapeutic response elicited by either or both the purified dsRNA or secondary therapeutic agent alone.
All U.S. patents, U.S. patent application publications, U.S. patent applications, foreign patents, foreign patent applications, non-patent publications, figures, tables, and websites referred to in this specification are expressly incorporated herein by reference, in their entirety.
EXAMPLES
EXAMPLE 1
Hydroxymethyl Nucleomonomer Substitution Patterns in RNA Complexes
Incorporation of hydroxymethyl nucleomonomers (e.g., monomer D) in specific positions in an RNA complex affects the gene silencing activity, cytokine induction, strand activity, "off- target" effects, thermal stability of the RNA complex, and in the case of Dicer substrate RNA complexes, Dicer processing of the RNA complex.
Example substitution patterns of hydroxymethyl nucleomonomers in a RISC RNA complex and Dicer RNA complex are provided below. The number of nucleomonomers of each strand of an RNA complex (double-stranded RNA) is represented (i.e., sequence independent) by a string of X's or H's. Each "X" independently and for each occurrence may be any nucleoside (e.g., adenine, guanine, cytosine, uracil, thymine, or any analog or derivative thereof), while each "H" independently and for each occurrence may be a non-nucleotide hydroxymethyl nucleomonomer (e.g., monomer D with any nucleobase). In each case, the sense strand and antisense strand anneal to form a double stranded region due to base pairing between each strand. The purpose of these diagrams is to show the substitution patterns of RNA complexes with hydroxymethyl nucleomonomers independent of sequence.
Any of the substitution patterns described herein may be applied to any RNA complex disclosed herein
Hydroxymethyl Nucleomonomer Substitution Patterns of a RISC RNA Complex
For each RNA complex below, the sense and antisense strand are each 21
nucleomonomers in length (except for Motif # P-l and P-1/G7 where the sense strand is 22 nucleomonomers in length) comprising either nucleosides or non-nucleotide hydroxymethyl nucleomonomers (e.g., monomer D). Each complex is identified with a "Motif #," and the position of the hydroxymethyl nucleomonomer(s), or "H", is provided. The position of each "H" in each strand is determined by counting from the 5 '-end of the strand in which the
hydroxymethyl nucleomonomer(s) is located. For any RNA complex disclosed herein, position - 1 (minus 1) or position 1 indicates that the hydroxymethyl nucleomonomer is the 3'-most nucleomonomer of that strand (or the last nucleomonomer at the 3 '-end of that strand). For the RISC length RNA complexes below, positions 21 and 22 of either the sense or antisense strand indicates that the nucleomonomers occupy the last two positions of that strand counting from the 5 '-end of the strand.
Motif # RNA Complex Strand Position(s)
5' XXXXXXXXXXXXXXXXXHXXX 3' SENSE 18
22
3' XXXXXXXXXXXXXXXXXXXXX 5' ANTISENSE 5' XHXXXXXXXXXXXXXXXXXXX 3' SENSE 2
24
3' HHXXXXXXXXXXXXXXXXXXH ANTISENSE 1,20,21
5' XXXXXXXXXXXXXXXXXXXHH 3' SENSE 20,21
G2
3' HHXXXXXXXXXXXXXXXXXHX 5' ANTISENSE 2, 20,21
5' XXXXXXXXXXXXXXXXXXXHH 3' SENSE 20,21
G3
3" HHXXXXXXXXXXXXXXXXHXX 5' ANTISENSE 3,20,21
XXXXXXXXXXXXXXXXXXXHH 20,21
G5
HHXXXXXXXXXXXXXXHXXXX 5, 20, 21
XXXXXXXXXXXXXXXXXXXHH 20,21
G6
HHXXXXXXXXXXXXXHXXXXX 6, 20,21
XXXXXXXXXXXXXXXXXXXHH 20,21
G7
HHXXXXXXXXXXXXHXXXXXX 7, 20,21
XXXXXXXXXXXXXXXXXXXHH 20,21
G8
HHXXXXXXXXXXXHXXXXXXX 8,20,21
5' XXXXXXXXXXXXXXXXXXXHH 3' SENSE 20,21
G10
3' HHXXXXXXXXXHXXXXXXXXX 5' ANTISENSE 10, 20, 21
5' XXXXXXXXXXXXXXXXXXXHH 3' SENSE 20, 21
G15
3' HHXXXXHXXXXXXXXXXXXXX 5' ANTISENSE 15,20,21
5' HXXXXXXXXXXXXXXXXXXXHH 3' SENSE -1,20,21
P-l
3' HHXXXXXXXXXXXXXXXXXXX 5' ANTISENSE 20,21
HXXXXXXXXXXXXXXXXXXHH 1,20,21
PI HHXXXXXXXXXXXXXXXXXXX 5' 20,21
XHXXXXXXXXXXXXXXXXXHH 2, 20, 21
P2
HHXXXXXXXXXXXXXXXXXXX 20,21
XXHXXXXXXXXXXXXXXXXHH 3,20,21
P3
HHXXXXXXXXXXXXXXXXXXX 20,21 5' XHXXXXXXXXXXXXXXXXXHH 3' SENSE 2, 20,21 P2/G2
3' HHXXXXXXXXXXXXXXXXXHX 5' ANTISENSE 2, 20, 21
5' HXXXXXXXXXXXXXXXXXXXHH 3' SENSE -1, 20, 21 P-1/G7
3' HHXXXXXXXXXXXXHXXXXXX 5' ANTISENSE 7, 20, 21
Hydroxymethyl Nucleomonomer Substitution Patterns of a Dicer RNA Complex
For each RNA complex below, the sense is 25 nucleomonomers in length and the antisense strand is 27 nucleomonomer is length (25/27-mer) comprising either nucleosides or non-nucleotide hydroxymethyl nucleomonomers (e.g., monomer D). Each complex is identified with a "Motif #" and the position of the hydroxymethyl nucleomonomer(s), or "H", is provided. The position of each "H" in each strand is determined by counting from the 5 '-end of the strand in which the hydroxymethyl nucleomonomer(s) is located.
RNA complexes having motif 10 have one blunt-ended and a 25 base pair duplex region with two non-nucleotide hydroxymethyl nucleomonomers attached to 5 '-end of the antisense strand (or at positions 26 and 27 in the antisense strand counting from the 5'-end of the antisense strand; the hydroxymethyl nucleomonomers occupy the last two positions of that strand counting from the 5 '-end of the strand ), and one non-nucleotide hydroxymethyl nucleomonomer attached to 3 '-end of the sense strand (or at position 25 in the sense strand counting from the 5 '-end of the sense strand; the hydroxymethyl nucleomonomer occupies the last position of that strand counting from the 5 '-end of the strand). Motif # RNA Complex Strand Position(s)
5' XXXXXXXXXXXXXXXXXXXXHHXXX 3' SENSE 21, 22
2
3' XXXXXXXXXXXXXXXXXXXXHHXXXXX 5' ANTISENSE 6, 7
5' XXXXXXXXXXXXXXXXXXXXHHXXX 3' SENSE 21, 22
3' XXXXXXXXXXXXXXXXXXXXXXXXXXX 5' ANTISENSE
5' XXXXXXXXXXXXXXXXXXXXXXXXX 3' SENSE
3' XXXXXXXXXXXXXXXXXXXXHHXXXXX 5' ANTISENSE 6, 7
5' XHXXXHXXXXXXXXXXXXXXXXXXX 3' SENSE 2, 6
3' XXXXXXXXXXXXXXXXXXXXXXXXXXX 5' ANTISENSE
5' XXXXXXXXXXXXXXXXXXXXXXXXX 3' SENSE
3' XXXXXXXXXXXXXXXHXXXHXXXXXXX 5' ANTISENSE 8, 12
5' XXXXXXXXXXXXXXXXXXXXXXXXX 3' SENSE
9
3' XXXXXXXXXXXXXXXHXXXXXXXXXXX 5' ANTISENSE 12
XXXXXXXXXXXXXXXXXXXXXXXXH 3' SENSE
HHXXXXXXXXXXXXXXXXXXXXXXXXX 5' ANTISENSE
EXAMPLE 2
HRAS RNA Complexes
The substitution patterns (motifs) represented in the example above were applied to different sequence specific RISC length RNA complexes. These RNA complexes are provided in table 1 below. Hydroxymethyl substituted monomer(s) in the sequences of the table below are identified as "unaH" where H is the one letter code for the nucleobase (e.g., "unaC" indicates that the cytosine comprises a hydroxymethyl substituted monomer).
Table 1. RNA Complexes that Target HRAS
RNA
Complex Sense Sequence Antisense Sequence
Identifier 5' to 3' orientation 5' to 3' orientation
(Motif#)
HRAS- 140 GGCAGGAGACCCUGUAGGA UCCUACAGGGUCUCCUGCC
Unmodified (SEQ ID NO:2) (SEQ ID NO:3)
HRAS- 140 GGCAGGAGACCCUGUAGGAUU UCCUACAGGGUCUCCUGCCUU UU ends (SEQ ID NO:4) (SEQ ID NO:5)
HRAS- 140 GGCAGGAGACCCUGUAGGAunaUunaU UCCUACAGGGUCUCCUGCCunaUunaU (31) (SEQ ID NO:6) (SEQ ID NO:7)
HRAS -140 unaUGGCAGGAGACCCUGUAGGA unaUunaU UCCUACAGGGUCUCCUGCCunaUunaU (P-1) (SEQ ID NO:8) (SEQ ID NO:7)
HRAS -140 unaGGCAGGAGACCCUGUAGGAunaUunaU UCCUACAGGGUCUCCUGCCunaUunaU (PI) (SEQ ID NO:9) (SEQ ID NO:7)
HRAS -140 GunaGCAGGAGACCCUGUAGGAunaUunaU UCCUACAGGGUCUCCUGCCunaUunaU (P2) (SEQ ID NO: 10) (SEQ ID NO:7)
HRAS -140 GGunaCAGGAGACCCUGUAGGAunaUunaU UCCUACAGGGUCUCCUGCCunaUunaU (P3) (SEQ ID NO: 11) (SEQ ID NO:7)
HRAS -140 GGCAGGAGACCCUGUAGGAunaUunaU UCCUACunaAGGGUCUCCUGCCunaUunaU (G7) (SEQ ID NO: 12) (SEQ ID NO: 13)
HRAS -140 unaUGGCAGGAGACCCUGUAGGAunaUunaU UCCUACunaAGGGUCUCCUGCCunaUunaU (P-1/G7) (SEQ ID NO: 14) (SEQ ID NO: 13)
HRAS -244 UGACCAUCCAGCUGAUCCA (SEQ ID NO: 15) UGGAUCAGCUGGAUGGUCA
Unmodified (SEQ ID NO: 16)
HRAS-244 UGACCAUCCAGCUGAUCCAUU UGGAUCAGCUGGAUGGUCAUU UU ends (SEQ ID NO: 17) (SEQ ID NO: 18)
HRAS -244 UGACCAUCCAGCUGAUCCAunaUunaU UGGAUCAGCUGGAUGGUCAunaUunaU (31) (SEQ ID NO: 19) (SEQ ID NO:20)
HRAS -244 unaUUGACCAUCCAGCUGAUCCAunaUunaU UGGAUCAGCUGGAUGGUCAunaUunaU
HRAS -337 CunaGUGCCUGUUGGACAUCCUunaUunaU AGGAUGUCCAACAGGCACGunaUunaU (P2) (SEQ ID NO:62) (SEQ ID NO:59)
HRAS -337 CGunaUGCCUGUUGGACAUCCUunaUunaU AGGAUGUCCAACAGGCACGunaUunaU (P3) (SEQ ID NO:63) (SEQ ID NO:59)
HRAS -337 CGUGCCUGUUGGACAUCCUunaUunaU AGGAUGunaUCCAACAGGCACGunaUunaU (G7) (SEQ ID NO:64) (SEQ ID NO:65)
HRAS -337 unaUCGUGCCUGUUGGACAUCCUunaUunaU AGGAUGunaUCCAACAGGCACGunaUunaU (P-1/G7) (SEQ ID NO:66) (SEQ ID NO:65)
HRAS -385 CCAUGCGGGACCAGUACAU AUGUACUGGUCCCGCAUGG
Unmodified (SEQ ID NO:67) (SEQ ID NO:68)
HRAS-385 CCAUGCGGGACCAGUACAUUU AUGUACUGGUCCCGCAUGGUU UU ends (SEQ ID NO:69) (SEQ ID NO:70)
HRAS -385 CCAUGCGGGACCAGUACAUunaUunaU AUGUACUGGUCCCGCAUGGunaUunaU (31) (SEQ ID NO:71) (SEQ ID NO:72)
HRAS -385 unaU CCAUGCGGGACCAGUACAUunaUunaU AUGUACUGGUCCCGCAUGGunaUunaU (P-l) (SEQ ID NO:73) (SEQ ID NO:72)
HRAS -385 una CCAUGCGGGACCAGUACAUunaUunaU AUGUACUGGUCCCGCAUGGunaUunaU (PI) (SEQ ID NO:74) (SEQ ID NO:72)
HRAS -385 CunaCAUGCGGGACCAGUACAUunaUunaU AUGUACUGGUCCCGCAUGGunaUunaU (P2) (SEQ ID NO:75) (SEQ ID NO:72)
HRAS -385 CCunaAUGCGGGACCAGUACAUunaUunaU AUGUACUGGUCCCGCAUGGunaUunaU (P3) (SEQ ID NO:76) (SEQ ID NO:72)
HRAS -385 CCAUGCGGGACCAGUACAUunaUunaU AUGUACunaUGGUCCCGCAUGGunaUunaU (G7) (SEQ ID NO:77) (SEQ ID NO:78)
HRAS -385 unaUCCAUGCGGGACCAGUACAUunaUunaU AUGUACunaUGGUCCCGCAUGGunaUunaU (P-1/G7) (SEQ ID NO:79) (SEQ ID NO:78)
HRAS -390 CGGGACCAGUACAUGCGCA UGCGCAUGUACUGGUCCCG
Unmodified (SEQ ID NO:80) (SEQ ID NO:81)
HRAS-390 CGGGACCAGUACAUGCGCAUU UGCGCAUGUACUGGUCCCGUU UU ends (SEQ ID NO:82) (SEQ ID NO:83)
HRAS -390 CGGGACCAGUACAUGCGCAunaUunaU UGCGCAUGUACUGGUCCCGunaUunaU (31) (SEQ ID NO:84) (SEQ ID NO:85)
HRAS -390 unaUCGGGACCAGUACAUGCGCAunaUunaU UGCGCAUGUACUGGUCCCGunaUunaU (P-l) (SEQ ID NO.-86) (SEQ ID NO:85)
HRAS -390 unaCGGGACCAGUACAUGCGCAunaUunaU UGCGCAUGUACUGGUCCCGunaUunaU (PI) (SEQ ID NO:87) (SEQ ID NO:85)
HRAS -390 CunaGGGACCAGUACAUGCGCAunaUunaU UGCGCAUGUACUGGUCCCGunaUunaU (P2) (SEQ ID NO:88) (SEQ ID NO:85)
HRAS -390 CGunaGGACCAGUACAUGCGCAunaUunaU UGCGCAUGUACUGGUCCCGunaUunaU (P3) (SEQ ID NO:89) (SEQ ID NO:85)
HRAS -390 CAGUGUUUCUUCUGCUUCAunaUunaU UGCGCAunaUGUACUGGUCCCGunaUunaU (G7) (SEQ ID NO:90) (SEQ ID NO:91)
HRAS -390 unaUCGGGACCAGUACAUGCGCAunaUunaU UGCGCAunaUGUACUGGUCCCGunaUunaU (P-1/G7) (SEQ ID NO:92) (SEQ ID NO:91)
HRAS -423 CUGUGUGUGUUUGCCAUCA UGAUGGCAAACACACACAG
Unmodified (SEQ ID NO:93) (SEQ ID NO:94)
HRAS-423 CUGUGUGUGUUUGCCAUCAUU UGAUGGCAAACACACACAGUU UU ends (SEQ ID NO:95) (SEQ ID NO:96)
HRAS -423 CUGUGUGUGUUUGCCAUCAunaUunaU UGAUGGCAAACACACACAGunaUunaU (31) (SEQ ID NO:97) (SEQ ID NO:98)
HRAS -423 unaUCUGUGUGUGUUUGCCAUCAunaUunaU UGAUGGCAAACACACACAGunaUunaU (P-l) (SEQ ID NO:99) (SEQ ID NO:98)
HRAS -423 unaCUGUGUGUGUUUGCCAUCAunaUunaU UGAUGGCAAACACACACAGunaUunaU (PI) (SEQ ID NO: 100) (SEQ ID NO:98)
HRAS -423 CunaUGUGUGUGUUUGCCAUCAunaUunaU UGAUGGCAAACACACACAGunaUunaU (P2) (SEQ ID NO: 101) (SEQ ID NO:98)
HRAS -423 CUunaGUGUGUGUUUGCCAUCAunaUunaU UGAUGGCAAACACACACAGunaUunaU
HRAS -472 AGUACAGGGAGCAGAUCAA UUGAUCUGCUCCCUGUACU
Unmodified (SEQ ID NO: 185) (SEQ ID NO: 186)
HRAS-472 AGUACAGGGAGCAGAUCAAUU UUGAUCUGCUCCCUGUACUUU UU ends (SEQ ID NO: 187) (SEQ ID NO: 188)
HRAS -472 AGUACAGGGAGCAGAUCAAunaUunaU UUGAUCUGCUCCCUGUACUunaUunaU (31) (SEQ ID NO: 189) (SEQ ID NO: 190)
HRAS -472 unaU AGUACAGGGAGCAGAUCAAunaUunaU UUGAUCUGCUCCCUGUACUunaUunaU (P-l) (SEQ ID NO: 191) (SEQ ID NO: 190)
HRAS -472 unaAGUACAGGGAGCAGAUCAAunaUunaU UUGAUCUGCUCCCUGUACUunaUunaU (PI) (SEQ ID NO: 192) (SEQ ID NO: 190)
HRAS - 472 AunaGUGGCACCAGAGGUGCUUunaUunaU UUGAUCUGCUCCCUGUACUunaUunaU (P2) (SEQ ID NO: 193) (SEQ ID NO: 190)
HRAS -472 AGunaUGGCACCAGAGGUGCUUunaUunaU UUGAUCUGCUCCCUGUACUunaUunaU (P3) (SEQ ID NO: 194) (SEQ ID NO: 190)
HRAS -472 AGUACAGGGAGCAGAUCAAunaUunaU UUGAUCunaUGCUCCCUGUACUunaUunaU (G7) (SEQ ID NO: 195) (SEQ ID NO: 196)
HRAS -472 unaU AGUACAGGGAGCAGAUCAAunaUunaU UUGAUCunaUGCUCCCUGUACUunaUunaU (P-1/G7) (SEQ ID NO: 197) (SEQ ID NO: 196)
HRAS -695 GAAGCUGAACCCUCCUGAU AUCAGGAGGGUUCAGCUUC
Unmodified (SEQ ID NO: 198) (SEQ ID NO: 199)
HRAS-695 GAAGCUGAACCCUCCUGAUUU AUCAGGAGGGUUCAGCUUCUU UU ends (SEQ ID NO:200) (SEQ ID NO:201)
HRAS -695 GAAGCUGAACCCUCCUGAUunaUunaU AUCAGGAGGGUUCAGCUUCunaUunaU (31) (SEQ ID NO:202) (SEQ ID NO:203)
HRAS -695 unaUGAAGCUGAACCCUCCUGAUunaUunaU AUCAGGAGGGUUCAGCUUCunaUunaU (P-l) (SEQ ID NO:204) (SEQ ID NO: 203)
HRAS -695 unaGAAGCUGAACCCUCCUGAUunaUunaU AUCAGGAGGGUUCAGCUUCunaUunaU (PI) (SEQ ID NO:205) (SEQ ID NO: 203)
HRAS - 695 GunaAAGCUGAACCCUCCUGAUunaUunaU AUCAGGAGGGUUCAGCUUCunaUunaU (P2) (SEQ ID NO.-206) (SEQ ID NO: 203)
HRAS -695 GAunaAGCUGAACCCUCCUGAUunaUunaU AUCAGGAGGGUUCAGCUUCunaUunaU (P3) (SEQ ID NO:207) (SEQ ID NO: 203)
HRAS -695 GAAGCUGAACCCUCCUGAUunaUunaU AUCAGGunaAGGGUUCAGCUUCunaUunaU (G7) (SEQ ID NO:208) (SEQ ID NO:209)
HRAS -695 unaUGAAGCUGAACCCUCCUGAUunaUunaU AUCAGGunaAGGGUUCAGCUUCunaUunaU (P-1/G7) (SEQ ID NO:210) (SEQ ID NO:209)
HRAS -698 GCUGAACCCUCCUGAUGAG CUCAUCAGGAGGGUUCAGC
Unmodified (SEQ ID NO:211) (SEQ ID NO:212)
HRAS-698 GCUGAACCCUCCUGAUGAGUU CUCAUCAGGAGGGUUCAGCUU UU ends (SEQ ID NO:213) (SEQ ID NO:214)
HRAS -698 GCUGAACCCUCCUGAUGAGunaUunaU CUCAUCAGGAGGGUUCAGCunaUunaU (31) (SEQ ID NO:215) (SEQ ID NO: 16)
HRAS -698 unaUGCUGAACCCUCCUGAUGAGunaUunaU CUCAUCAGGAGGGUUCAGCunaUunaU (P-l) (SEQ ID NO:217) (SEQ ID NO:216)
HRAS -698 unaGCUGAACCCUCCUGAUGAGunaUunaU CUCAUCAGGAGGGUUCAGCunaUunaU (PI) (SEQ ID NO:218) (SEQ ID NO:216)
HRAS - 698 GunaCUGAACCCUCCUGAUGAGunaUunaU CUCAUCAGGAGGGUUCAGCunaUunaU (P2) (SEQ ID NO:219) (SEQ ID NO:216)
HRAS -698 GCunaUGAACCCUCCUGAUGAGunaUunaU CUCAUCAGGAGGGUUCAGCunaUunaU (P3) (SEQ ID NO:220) (SEQ ID NO:216)
HRAS -698 GCUGAACCCUCCUGAUGAGunaUunaU CUCAUCunaAGGAGGGUUCAGCunaUunaU (G7) (SEQ ID NO.-221) (SEQ ID NO.-222)
HRAS -698 unaUGCUGAACCCUCCUGAUGAGunaUunaU CUCAUCunaAGGAGGGUUCAGCunaUunaU (P-1/G7) (SEQ ID NO:223) (SEQ ID NO:222)
HRAS -701 GAACCCUCCUGAUGAGAGU ACUCUCAUCAGGAGGGUUC
Unmodified (SEQ ID NO:224) (SEQ ID NO:225)
Gene Silencing Activity of HRAS RNA Complexes
The gene silencing activity (or "knockdown activity") of RNA complexes that target the HRAS mRNA was examined in vitro in KU-7 cells (bladder cancer cell line).
Briefly, cells were plated at a density of 10,000 cells/well on a 96-well plate. Twenty- four hours later, 25 μΐ. mixture containing 5 nM siRNA and RNAi MAX diluted 1/50 in optiMEM media was added to each well containing 100 μΙ_. cell medium with 10% fetal bovine serum. The transfection was performed in triplicate. The transfection mixture was incubated with the cells for 24 hours. Following the incubation, cells were lysed, RNA extracted and qRT- PCR was performed to determine gene expression levels. A scrambled sequence (Qneg;
QIAGEN) served as the negative control RNA complex. Knockdown activity in transfected and untransfected cells was normalized to the negative control RNA complex and presented as a normalized value of the Qneg RNA complex negative control (i.e., no HRAS knockdown was represented as 0%, thus a higher percentage indicates greater knockdown).
The following results were obtained as shown in Table 2.
The results show that the HRAS RNA complexes reduced HRAS mRNA expression in KU-7 bladder cancer cells. Sixteen of the 22 RNA complexes showed greater than 90% KD at 5 nM relative to the negative control RNA complex.
The gene silencing activity of hydroxymethyl substituted HRAS RNA complex HRAS- 429(P-1) was examined at a concentration ranging from 0.008 nM to 25 nM in KU-7 cells. Cell viability was also examined in KU-7 cells for each concentration. For gene silencing activity, cells were transfected according to the protocol provided above. For cell viability, KU-7 cells were plated at a density of 5,000 cells/well and transfected with RNAi MAX. Cell viability was measured with the CELLTITER 96 assay kit (PROMEGA) per the manufacturer's protocol. A scrambled sequence of a Survivin siRNA (Srv-scr; DX10103) served as the negative control RNA complex (shown below). Knockdown activity and cell viability in transfected cells were normalized to the negative control Srv-scr RNA complex and presented as a normalized value of the Srv-scr RNA complex negative control (i.e., greater knockdown is represented by a higher percentage, and reduced cell viability is represented by a lower percentage).
Srv-scr (DX10103) - negative control:
Sense Strand: 5'- UCCCGUUCUAGUGUUUCCUunaUunaU - 3' (SEQ ID
NO:290)
Antisense Strand: 5'- AGGAAACACUAGAACGGGAunaUunaU - 3' (SEQ ID
NO:291)
The following results were obtained as shown in Table 3.
Table 3. HRAS mRNA Knockdown in Bladder Cancer Cells
The results show that the HRAS-429 (P-l) RNA complex reduced HRAS mRNA expression in KU-7 bladder cancer cells in a dose dependent manner relative to the scrambled negative control RNA complex. Further, the results show that the HRAS-429 (P-l) RNA complex reduced cell viability in a dose dependent manner relative to the scrambled negative
control RNA complex at RNA concentrations greater than 0.2nM. No significant reduction in cell viability was observed at RNA concentrations of 0.2nM or below (i.e., at concentrations for which % knockdown was less than 50%). EXAMPLE 3
Gene Silencing Activity of HRAS RNA Complexes Delivered to Cells with a DILA2
Nanoparticle Delivery Formulation
The gene silencing activity (or "knockdown activity") of RNA complexes that target the HRAS mRNA was examined in vitro in KU-7 cells (bladder cancer cell line) by transfection with a DILA2 Nanoparticle formulation.
Briefly, cells were plated at a density of 10,000 cells/well on a 96- well plate. Twenty- four hours later, 25 μΙ_. mixture containing 25 nM HRAS-429 (P-1) RNA complex and a DILA2 nanoparticle delivery formulation (C18: l-norArg-C16/ CHEMS/CHOL/DMPE-PEG2K
(45:28:25:2); N/P 1.4) was added to each well containing 100 μΙ_ cell medium with 10% fetal bovine serum. The transfection was performed in triplicate. The transfection mixture was incubated with the cells for 24 hours. Following the incubation, cells were lysed, RNA extracted and qRT-PCR was performed to determine gene expression levels.
Srv-scr served as the negative control RNA complex. Knockdown activity was normalized to the negative control Srv-scr RNA complex and presented as a normalized value of the Srv-scr RNA complex negative control (i.e., greater knockdown is represented by a higher percentage). Cell viability was also assessed using the methods described above.
The results showed that the HRAS-429 (P-1) RNA complex (25 nM) formulated with the DILA2 nanoparticle delivery formulation reduced HRAS mRNA expression by 96% in KU-7 bladder cancer cells relative to the scrambled negative control RNA complex. No reduction in cell viability was observed relative to the negative control.
EXAMPLE 4
HRAS RNA Complexes Reduced Tumor Growth in Mice
The effect of HRAS siRNA on tumor growth in an orthotopic bladder cancer mouse model was examined.
For each of the two treatment groups, eleven eight-week old female athymic nude mice were anaesthetized with 1.75% isoflurane prior to the implantation of KU-7-LUC cells (Human bladder cancer cells constitutively expressing luciferase). A superficial 4/0 silk purse-string suture was placed around the urethral meatus before a lubricated catheter was inserted through
the urethra into the bladder. The bladder was washed once with PBS prior to instilling approximately two million KU-7-LUC cells in 50 μΐ- volume into the bladder. A suture was used to occlude the urethra for 2 hours.
The KU-7-LUC orthotopic bladder cancer bearing mice were treated with unmodified and modified HRAS RNA complex (HRAS-429 (P-l)) or a control RNA complex (Srv-scr). Each mouse was dosed with 1.0 mg/kg of RNA complex and a DFLA2 nanoparticle delivery formulation (C18: l-norArg-C 16/ CHEMS/CHOL DMPE-PEG2K (45:28:25:2); N/P 1.4) at a total volume of 50 μί, twice weekly on days 2, 4, 7 and 9 post tumor inoculation. Each mouse was dosed with the RNA complex and DILA2 nanoparticle delivery formulation by catheter directly to the bladder. RNA complex DILA2 nanoparticle delivery formulations were prepared as disclosed herein.
Tumor progression was assessed on days 1, 6, 10, 15, 21, 28, 36, and 43 by an intraperitoneal injection of 150 mg/kg luciferin post anesthesia in the supine position followed by image acquisition by an IVIS (inv vivo imaging system) 200 system (XENOGEN). Tumor size for each mouse was determined by quantifying bioluminescence (i.e., photons/second) and directly correlating the degree of bioluminescity with tumor size (i.e., greater intensity indicates the presence of a larger tumor).
The tumor bioluminescence results are shown in Table 4 and in Figs. 4a and 4b.
Bladder tumor volume was also examined. Change in bladder tumor volume was expressed both as a fold change and percent change relative to the negative control. A fold change above one indicates that the tumor volume has decreased relative to the tumor volume observed in the mice treated with the negative control. A higher percentage indicates a greater decrease in tumor volume relative to the tumor volume observed in the mice treated with the negative control. The results for day 21 post tumor implantation are shown in Table 5.
These results show that at the 1.0 mg/kg dosage, the HRAS (P-1) RNA complex reduced the tumor volume by approximately 4 fold by day 21 post tumor implantation compared to the negative control Srv-scr RNA complex. Expressed as a percent reduction of tumor volume, the HRAS-429 (P-1) complex reduced tumor volume by about 76% at day 21 post tumor implantation as compared to the negative control Srv-scr RNA complex.
EXAMPLE 5
HRAS RNA Complexes Reduced HRAS mRNA Expression In Bladder Tumors of Mice HRAS gene expression levels in the tumors were also measured using total bladder RNA harvested at day 21. Briefly, the procedures and protocol described in Example 4 were used to prepare orthotopic bladder cancer bearing mice. Groups of mice were dosed with RNA complexes and DILA2 nanoparticle delivery formulation as described above. Total bladder RNA was extracted from the mice at day 21, and qRT-PCR was performed to determine HRAS gene expression levels. Expression levels of HRAS in tumors from all groups were normalized to TATA Binding Protein (TBP) and HPRT1 mRNA expression levels in tumor cells.
Knockdown activity in tumors from each non-control group was normalized to the knockdown activity of the corresponding negative control (i.e., Srv-scr) and presented as a normalized value of the negative control (i.e., no HRAS knockdown was represented as 0%, thus a higher percentage indicates greater knockdown).
Table 6 indicates HRAS mRNA levels and knockdown activity in tumors of mice treated with 1.0 mg/kg HRAS-429 (P-1) RNA complex, normalized to the negative control (i.e., tumors from group dosed with 1 mg/kg Srv-scr RNA complex as described in Example 4).
These results indicate that HRAS-429 (P-l) RNA complex reduced HRAS mRNA levels in the tumors by 61 % relative to the negative control. The teachings of all of references cited herein including patents, patent applications, journal articles, web pages, tables, and priority documents are incorporated herein in their entirety by reference. Although the foregoing disclosure has been described in detail by way of example for purposes of clarity of understanding, it will be apparent to the artisan that certain changes and modifications may be practiced within the scope of the appended claims which are presented by way of illustration not limitation. In this context, various publications and other references have been cited within the foregoing disclosure for economy of description. It is noted, however, that the various publications discussed herein are incorporated solely for their disclosure prior to the filing date of the present application, and the inventors reserve the right to antedate such disclosure by virtue of prior invention.
Claims
WHAT IS CLAIMED IS:
I . A nucleic acid that down regulates the expression of a v-Ha-ras Harvey rat sarcoma viral oncogene homolog gene (HRAS) mRNA, the nucleic acid comprising an antisense strand having a region of 15 to 60 contiguous nucleomonomers, wherein at least 15 contiguous nucleomonomers of the nucleic acid correspond to 15 contiguous nucleomonomers of one or more of SEQ ID NOs: 3, 16, 29, 42, 55, 68, 81 , 94, 107, 120, 133, 146, 159, 172, 186, 199, 212, 225, 239, 252, 265, and 278, and a sense strand complementary to the antisense strand, wherein the antisense strand and the sense strand can anneal to form a double-stranded region of 15 base pairs to 60 base pairs.
2. The nucleic acid of claim 1, wherein the nucleic acid is a ribonucleic acid.
3. The nucleic acid of claim 2, wherein the ribonucleic acid is a siRNA.
4. The nucleic acid of claim 1 wherein the antisense strand is from 18 to 25 nucleomonomers in length.
5. The nucleic acid of claim 1 wherein the sense strand is a contiguous strand of nucleomonomers.
6. The nucleic acid of claim 1 wherein the sense strand has one or more nicks.
7. The nucleic acid of claim 1 wherein the sense strand has one or more gaps.
8. The nucleic acid of claim 7 wherein the one or more gaps, independently for each occurrence, comprise from 1 to 10 unpaired nucleomonomers.
9. The nucleic acid of claim 1 wherein the nucleic acid has a blunt end.
10. The nucleic acid of claim 1 further comprising a 3 '-end overhang.
I I . The nucleic acid of claim 1 further comprising at least one hydroxymethyl substituted nucleomonomer.
12. The nucleic acid of claim 11 wherein the at least one hydroxymethyl substituted nucleomonomer is selected from:
Monomer D Monomer F Monomer G
Monomer J wherein,
R is selected from the group consisting of hydrogen, a methyl group, C(l-10) alkyl, cholesterol, naturally or non-naturally occurring amino acid, sugar, vitamin, fluorophore, polyamine and fatty acid; and
Base is a nucleobase or analog thereof.
13. The nucleic acid of claim 12 wherein one or more of the at least one
hydroxymethyl substituted nucleomonomers of the nucleic acid further comprises a 2'-0-methyl modification.
14. The nucleic acid of claim 12 wherein one or both of the last two positions at the 3 '-end of the sense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer.
15. The nucleic acid of claim 12 wherein one or both of the last two positions at the 3'-end of the antisense strand are occupied by the same or different hydroxymethyl substituted nucleomonomer.
16. The nucleic acid of claim 12 wherein any one or more of the last three positions at the 5 '-end of the sense strand is occupied by the same or different hydroxymethyl substituted nucleomonomer.
17. The nucleic acid of claim 12 wherein at least one hydroxymethyl substituted nucleomonomer is in a double-stranded region of the nucleic acid.
18. The nucleic acid of claim 1 wherein one or more nucleotides of the nucleic acid further comprises a 2' -modification of the sugar of the one or more nucleotides.
19. The nucleic acid of claim 18 wherein the 2 '-modification of the sugar of the one or more nucleotides is a 2'-0-methyl modification.
20. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 5, 7 or 13.
21. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 18, 20 or 26.
22. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 31, 33 or 39.
23. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 44, 46 or 52.
24. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO:
57, 59 or 65.
25. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 70, 72 or 78.
26. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 83, 85 or 91.
27. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 96, 98 or 104.
28. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 109, 1 1 1 or 1 17.
29. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO:
122, 124 or 130.
30. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 135, 137 or 143.
31. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ID NO: 148, 150 or 156.
32. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO:
161 , 163 or 169.
33. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 174, 176 or 183.
34. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 188, 190 or 196.
35. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 201 , 203 or 209.
36. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 214, 216 or 222.
37. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO:
227, 229 or 236.
38. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 241, 243 or 249.
39. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 254, 256 or 262.
40. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 267, 269 or 275.
41. The nucleic acid of claim 12 wherein the antisense strand comprises SEQ ED NO: 280, 282 or 288.
42. Use of a nucleic acid as defined in any one of the preceding claims for the manufacture of a medicament for use in the therapy of cancer.
43. A method for reducing the expression of a human HRAS gene, comprising administering nucleic acid according to any one of claims 1-42 to a cell expressing a HRAS gene, wherein the nucleic acid reduces expression of the HRAS gene in the cell.
44. The method according to claim 43 wherein the cell is a human cell.
45. A method for treating or managing a disease or condition in a subject associated, linked, and/or resulting from aberrant HRAS gene expression, comprising administering to the subject in need of treatment or management a nucleic acid according to any one of claims 1-42, wherein the nucleic acid reduces the expression of the HRAS gene thereby treating or managing the disease or condition.
46. The method of claim 45, wherein the disease or condition is selected from one or more hyperproliferative diseases or disorders, leukemia, cutaneous melanoma, adenocarcinoma, squamous cell carcinoma, Philadelphia chromosome-negative myeloproliferative disorder, myelodysplastic syndrome, transitional cell carcinoma, ovarian cancer, brain tumors, breast cancer, bladder cancer, lung cancer, kidney tumors, urinary tract tumors, pancreatic carcinoma, and colorectal adenoma; as well as one or more angiogenic diseases or disorders, hepatocellular carcinoma (HCC), NSCLC (lung nonsmall cell lung cancer), melanoma, colon cancer, prostate cancer, and glioblastoma.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US32575710P | 2010-04-19 | 2010-04-19 | |
| US61/325,757 | 2010-04-19 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| WO2011133584A2 true WO2011133584A2 (en) | 2011-10-27 |
| WO2011133584A3 WO2011133584A3 (en) | 2012-01-12 |
| WO2011133584A8 WO2011133584A8 (en) | 2012-06-07 |
Family
ID=44121007
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/033102 WO2011133584A2 (en) | 2010-04-19 | 2011-04-19 | Nucleic acid compounds for inhibiting hras gene expression and uses thereof |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011133584A2 (en) |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017510583A (en) * | 2014-03-25 | 2017-04-13 | アークトゥラス・セラピューティクス・インコーポレイテッドArcturus Therapeutics,Inc. | Transthyretin allele-selective UNA oligomers for gene silencing |
| EP3122889A4 (en) * | 2014-03-25 | 2017-11-01 | Arcturus Therapeutics, Inc. | Una oligomers having reduced off-target effects in gene silencing |
| US10421964B2 (en) | 2015-07-23 | 2019-09-24 | Arcturus Therapeutics, Inc. | UNA oligomers and compositions for treating amyloidosis |
| US10519447B2 (en) | 2015-04-01 | 2019-12-31 | Arcturus Therapeutics, Inc. | Therapeutic UNA oligomers and uses thereof |
| WO2020016894A1 (en) * | 2018-07-19 | 2020-01-23 | Yeda Research And Development Co. Ltd. | Sphingosine analogs and use thereof against bacterial lung infections |
| US10604758B2 (en) | 2014-03-25 | 2020-03-31 | Arcturus Therapeutics, Inc. | Therapeutic oligomers for treating amyloidosis |
Citations (68)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1989002439A1 (en) | 1987-09-21 | 1989-03-23 | Ml Technology Ventures, L.P. | Non-nucleotide linking reagents for nucleotide probes |
| US4897355A (en) | 1985-01-07 | 1990-01-30 | Syntex (U.S.A.) Inc. | N[ω,(ω-1)-dialkyloxy]- and N-[ω,(ω-1)-dialkenyloxy]-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| WO1991003162A1 (en) | 1989-08-31 | 1991-03-21 | City Of Hope | Chimeric dna-rna catalytic sequences |
| WO1992007065A1 (en) | 1990-10-12 | 1992-04-30 | MAX-PLANCK-Gesellschaft zur Förderung der Wissenschaften e.V. | Modified ribozymes |
| US5208036A (en) | 1985-01-07 | 1993-05-04 | Syntex (U.S.A.) Inc. | N-(ω, (ω-1)-dialkyloxy)- and N-(ω, (ω-1)-dialkenyloxy)-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| WO1993015187A1 (en) | 1992-01-31 | 1993-08-05 | Massachusetts Institute Of Technology | Nucleozymes |
| US5264618A (en) | 1990-04-19 | 1993-11-23 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| WO1993023569A1 (en) | 1992-05-11 | 1993-11-25 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for inhibiting viral replication |
| US5279833A (en) | 1990-04-04 | 1994-01-18 | Yale University | Liposomal transfection of nucleic acids into animal cells |
| US5283185A (en) | 1991-08-28 | 1994-02-01 | University Of Tennessee Research Corporation | Method for delivering nucleic acids into cells |
| WO1994002595A1 (en) | 1992-07-17 | 1994-02-03 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for treatment of animal diseases |
| US5334711A (en) | 1991-06-20 | 1994-08-02 | Europaisches Laboratorium Fur Molekularbiologie (Embl) | Synthetic catalytic oligonucleotide structures |
| US5334761A (en) | 1992-08-28 | 1994-08-02 | Life Technologies, Inc. | Cationic lipids |
| WO1995006731A2 (en) | 1993-09-02 | 1995-03-09 | Ribozyme Pharmaceuticals, Inc. | Non-nucleotide containing enzymatic nucleic acid |
| WO1995011910A1 (en) | 1993-10-27 | 1995-05-04 | Ribozyme Pharmaceuticals, Inc. | 2'-amido and 2'-peptido modified oligonucleotides |
| US5505931A (en) | 1993-03-04 | 1996-04-09 | The Dow Chemical Company | Acid cleavable compounds, their preparation and use as bifunctional acid-labile crosslinking agents |
| WO1996010390A1 (en) | 1994-09-30 | 1996-04-11 | Inex Pharmaceuticals Corp. | Novel compositions for the introduction of polyanionic materials into cells |
| WO1996010392A1 (en) | 1994-09-30 | 1996-04-11 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| WO1996010391A1 (en) | 1994-09-30 | 1996-04-11 | The University Of British Columbia | Polyethylene glycol modified ceramide lipids and liposome uses thereof |
| US5563250A (en) | 1987-12-02 | 1996-10-08 | Neorx Corporation | Cleavable conjugates for the delivery and release of agents in native form |
| US5627053A (en) | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| WO1997026270A2 (en) | 1996-01-16 | 1997-07-24 | Ribozyme Pharmaceuticals, Inc. | Synthesis of methoxy nucleosides and enzymatic nucleic acid molecules |
| US5716824A (en) | 1995-04-20 | 1998-02-10 | Ribozyme Pharmaceuticals, Inc. | 2'-O-alkylthioalkyl and 2-C-alkylthioalkyl-containing enzymatic nucleic acids (ribozymes) |
| WO1998013526A1 (en) | 1996-09-26 | 1998-04-02 | Oligos Etc. Inc. | Three component chimeric antisense oligonucleotides |
| US5736392A (en) | 1995-06-07 | 1998-04-07 | Life Technologies, Inc. | Peptide-enhanced cationic lipid transfections |
| US5767264A (en) | 1993-01-22 | 1998-06-16 | Mta Zozponti Kemiai Kutato Intezet | Oligodeoxynucleotides containing 5-alkyl, 5-(1-alkenyl)- and 5-(1-alkynl) pyrimidines |
| US5785992A (en) | 1994-09-30 | 1998-07-28 | Inex Pharmaceuticals Corp. | Compositions for the introduction of polyanionic materials into cells |
| WO1999031262A2 (en) | 1997-12-16 | 1999-06-24 | Valentis, Inc. | Needle-free injection of formulated nucleic acid molecules |
| WO1999032619A1 (en) | 1997-12-23 | 1999-07-01 | The Carnegie Institution Of Washington | Genetic inhibition by double-stranded rna |
| WO1999054459A2 (en) | 1998-04-20 | 1999-10-28 | Ribozyme Pharmaceuticals, Inc. | Nucleic acid molecules with novel chemical compositions capable of modulating gene expression |
| US6001311A (en) | 1997-02-05 | 1999-12-14 | Protogene Laboratories, Inc. | Apparatus for diverse chemical synthesis using two-dimensional array |
| WO2000053722A2 (en) | 1999-03-10 | 2000-09-14 | Phogen Limited | Delivery of nucleic acids and proteins to cells |
| WO2001005374A1 (en) | 1999-07-15 | 2001-01-25 | Inex Pharmaceuticals Corp. | Methods for preparation of lipid-encapsulated therapeutic agents |
| US6200599B1 (en) | 1999-10-07 | 2001-03-13 | The Regents Of The University Of California | Ortho ester lipids |
| US6268490B1 (en) | 1997-03-07 | 2001-07-31 | Takeshi Imanishi | Bicyclonucleoside and oligonucleotide analogues |
| US6300074B1 (en) | 1990-06-11 | 2001-10-09 | Gilead Sciences, Inc. | Systematic evolution of ligands by exponential enrichment: Chemi-SELEX |
| US6376248B1 (en) | 1997-03-14 | 2002-04-23 | Life Technologies, Inc. | Peptide-enhanced transfections |
| US6426086B1 (en) | 1998-02-03 | 2002-07-30 | The Regents Of The University Of California | pH-sensitive, serum-stable liposomes |
| US20030130186A1 (en) | 2001-07-20 | 2003-07-10 | Chandra Vargeese | Conjugates and compositions for cellular delivery |
| US6670461B1 (en) | 1997-09-12 | 2003-12-30 | Exiqon A/S | Oligonucleotide analogues |
| US6733777B2 (en) | 1996-11-04 | 2004-05-11 | Qiagen Gmbh | Cationic reagents of transfection |
| US20040110296A1 (en) | 2001-05-18 | 2004-06-10 | Ribozyme Pharmaceuticals, Inc. | Conjugates and compositions for cellular delivery |
| US20040132161A1 (en) | 1998-04-20 | 2004-07-08 | Finkel Terri H. | Methods and compositions for increasing CD4lymphocyte immune responsiveness |
| US20040142025A1 (en) | 2002-06-28 | 2004-07-22 | Protiva Biotherapeutics Ltd. | Liposomal apparatus and manufacturing methods |
| US6835393B2 (en) | 1998-01-05 | 2004-12-28 | University Of Washington | Enhanced transport using membrane disruptive agents |
| US6843942B2 (en) | 2000-11-03 | 2005-01-18 | Polymun Scientific Immunobilogische Forschung Gmbh | Method and device for producing lipid vesicles |
| US6849272B1 (en) | 1999-04-21 | 2005-02-01 | Massachusetts Institute Of Technology | Endosomolytic agents and cell delivery systems |
| US20050064595A1 (en) | 2003-07-16 | 2005-03-24 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering RNA |
| US6897196B1 (en) | 2001-02-07 | 2005-05-24 | The Regents Of The University Of California | pH sensitive lipids based on ortho ester linkers, composition and method |
| US20060014289A1 (en) | 2004-04-20 | 2006-01-19 | Nastech Pharmaceutical Company Inc. | Methods and compositions for enhancing delivery of double-stranded RNA or a double-stranded hybrid nucleic acid to regulate gene expression in mammalian cells |
| US20060040882A1 (en) | 2004-05-04 | 2006-02-23 | Lishan Chen | Compostions and methods for enhancing delivery of nucleic acids into cells and for modifying expression of target genes in cells |
| US20060142230A1 (en) | 2003-08-25 | 2006-06-29 | Nastech Pharmaceutical Company Inc. | Double-stranded ribonucleic acid molecules having ribothymidine |
| US7098032B2 (en) | 2001-01-02 | 2006-08-29 | Mirus Bio Corporation | Compositions and methods for drug delivery using pH sensitive molecules |
| US7108863B2 (en) | 2001-03-26 | 2006-09-19 | Alza Corporation | Liposome composition for improved intracellular delivery of a therapeutic agent |
| US7138382B2 (en) | 1999-06-07 | 2006-11-21 | Mirus Bio Corporation | Compositions and methods for drug delivery using pH sensitive molecules |
| US20070252295A1 (en) | 2001-02-21 | 2007-11-01 | Steffen Panzner | Amphoteric liposomes |
| US20080055366A1 (en) | 2005-10-31 | 2008-03-06 | Trudy Benjamin | Fluid ejection device |
| US20080055360A1 (en) | 2001-03-27 | 2008-03-06 | Silverbrook Research Pty Ltd | Printhead assembly of printhead integrated circuit modules |
| US20080055383A1 (en) | 2004-10-07 | 2008-03-06 | Poncelet Olivier J | Inkjet Recording Element |
| US20080055385A1 (en) | 2006-09-04 | 2008-03-06 | Fujifilm Corporation | Ink Set and Image Forming Apparatus and Method |
| US20080055378A1 (en) | 2004-09-18 | 2008-03-06 | Drury Paul R | Fluid Supply Method and Apparatus |
| US20080055357A1 (en) | 2006-08-31 | 2008-03-06 | Canon Kabushiki Kaisha | Inkjet printing apparatus |
| US20080055370A1 (en) | 2000-03-27 | 2008-03-06 | Fuji Photo Film Co., Ltd. | Multi-nozzle ink jet head and manufacturing method thereof |
| US20080055386A1 (en) | 2006-08-29 | 2008-03-06 | Samsung Electronics Co., Ltd. | Medium supplying apparatus of image forming device |
| US20080055339A1 (en) | 2006-08-30 | 2008-03-06 | Marketech International Corp. | Method for automatically detecting and adjusting grayscale/white balance of display |
| US20080055365A1 (en) | 2002-11-23 | 2008-03-06 | Silverbrook Research Pty Ltd | Inkjet printhead with suspended heater elements |
| US20080064417A1 (en) | 2006-09-11 | 2008-03-13 | Research In Motion Limited | Apparatus, and associated method, for paging an access terminal in a radio communication system |
| US20080317839A1 (en) | 2007-05-04 | 2008-12-25 | Nastech Pharmaceutical Company Inc. | Amino acid lipids and uses thereof |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1390472A4 (en) * | 2001-05-29 | 2004-11-17 | Sirna Therapeutics Inc | Nucleic acid treatment of diseases or conditions related to levels of ras, her2 and hiv |
| CN104480112B (en) * | 2007-05-22 | 2018-06-12 | 阿克丘勒斯治疗公司 | For the UNA oligomer for the treatment of |
| WO2010017319A2 (en) * | 2008-08-05 | 2010-02-11 | Mdrna, Inc. | Nucleic acid compounds for inhibiting plk1 gene expression and uses thereof |
| US20110236972A1 (en) * | 2008-08-05 | 2011-09-29 | Marina Biotech, Inc. | Nucleic acid compounds for inhibiting birc5 gene expression and uses thereof |
-
2011
- 2011-04-19 WO PCT/US2011/033102 patent/WO2011133584A2/en active Application Filing
Patent Citations (71)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4897355A (en) | 1985-01-07 | 1990-01-30 | Syntex (U.S.A.) Inc. | N[ω,(ω-1)-dialkyloxy]- and N-[ω,(ω-1)-dialkenyloxy]-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| US5208036A (en) | 1985-01-07 | 1993-05-04 | Syntex (U.S.A.) Inc. | N-(ω, (ω-1)-dialkyloxy)- and N-(ω, (ω-1)-dialkenyloxy)-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| WO1989002439A1 (en) | 1987-09-21 | 1989-03-23 | Ml Technology Ventures, L.P. | Non-nucleotide linking reagents for nucleotide probes |
| US5563250A (en) | 1987-12-02 | 1996-10-08 | Neorx Corporation | Cleavable conjugates for the delivery and release of agents in native form |
| WO1991003162A1 (en) | 1989-08-31 | 1991-03-21 | City Of Hope | Chimeric dna-rna catalytic sequences |
| US5279833A (en) | 1990-04-04 | 1994-01-18 | Yale University | Liposomal transfection of nucleic acids into animal cells |
| US5264618A (en) | 1990-04-19 | 1993-11-23 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| US5459127A (en) | 1990-04-19 | 1995-10-17 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| US6300074B1 (en) | 1990-06-11 | 2001-10-09 | Gilead Sciences, Inc. | Systematic evolution of ligands by exponential enrichment: Chemi-SELEX |
| WO1992007065A1 (en) | 1990-10-12 | 1992-04-30 | MAX-PLANCK-Gesellschaft zur Förderung der Wissenschaften e.V. | Modified ribozymes |
| US5334711A (en) | 1991-06-20 | 1994-08-02 | Europaisches Laboratorium Fur Molekularbiologie (Embl) | Synthetic catalytic oligonucleotide structures |
| US5283185A (en) | 1991-08-28 | 1994-02-01 | University Of Tennessee Research Corporation | Method for delivering nucleic acids into cells |
| WO1993015187A1 (en) | 1992-01-31 | 1993-08-05 | Massachusetts Institute Of Technology | Nucleozymes |
| WO1993023569A1 (en) | 1992-05-11 | 1993-11-25 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for inhibiting viral replication |
| WO1994002595A1 (en) | 1992-07-17 | 1994-02-03 | Ribozyme Pharmaceuticals, Inc. | Method and reagent for treatment of animal diseases |
| US5334761A (en) | 1992-08-28 | 1994-08-02 | Life Technologies, Inc. | Cationic lipids |
| US5767264A (en) | 1993-01-22 | 1998-06-16 | Mta Zozponti Kemiai Kutato Intezet | Oligodeoxynucleotides containing 5-alkyl, 5-(1-alkenyl)- and 5-(1-alkynl) pyrimidines |
| US5505931A (en) | 1993-03-04 | 1996-04-09 | The Dow Chemical Company | Acid cleavable compounds, their preparation and use as bifunctional acid-labile crosslinking agents |
| WO1995006731A2 (en) | 1993-09-02 | 1995-03-09 | Ribozyme Pharmaceuticals, Inc. | Non-nucleotide containing enzymatic nucleic acid |
| WO1995011910A1 (en) | 1993-10-27 | 1995-05-04 | Ribozyme Pharmaceuticals, Inc. | 2'-amido and 2'-peptido modified oligonucleotides |
| US5627053A (en) | 1994-03-29 | 1997-05-06 | Ribozyme Pharmaceuticals, Inc. | 2'deoxy-2'-alkylnucleotide containing nucleic acid |
| WO1996010391A1 (en) | 1994-09-30 | 1996-04-11 | The University Of British Columbia | Polyethylene glycol modified ceramide lipids and liposome uses thereof |
| WO1996010392A1 (en) | 1994-09-30 | 1996-04-11 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| WO1996010390A1 (en) | 1994-09-30 | 1996-04-11 | Inex Pharmaceuticals Corp. | Novel compositions for the introduction of polyanionic materials into cells |
| US5753613A (en) | 1994-09-30 | 1998-05-19 | Inex Pharmaceuticals Corporation | Compositions for the introduction of polyanionic materials into cells |
| US5785992A (en) | 1994-09-30 | 1998-07-28 | Inex Pharmaceuticals Corp. | Compositions for the introduction of polyanionic materials into cells |
| US5716824A (en) | 1995-04-20 | 1998-02-10 | Ribozyme Pharmaceuticals, Inc. | 2'-O-alkylthioalkyl and 2-C-alkylthioalkyl-containing enzymatic nucleic acids (ribozymes) |
| US5736392A (en) | 1995-06-07 | 1998-04-07 | Life Technologies, Inc. | Peptide-enhanced cationic lipid transfections |
| WO1997026270A2 (en) | 1996-01-16 | 1997-07-24 | Ribozyme Pharmaceuticals, Inc. | Synthesis of methoxy nucleosides and enzymatic nucleic acid molecules |
| WO1998013526A1 (en) | 1996-09-26 | 1998-04-02 | Oligos Etc. Inc. | Three component chimeric antisense oligonucleotides |
| US6733777B2 (en) | 1996-11-04 | 2004-05-11 | Qiagen Gmbh | Cationic reagents of transfection |
| US6001311A (en) | 1997-02-05 | 1999-12-14 | Protogene Laboratories, Inc. | Apparatus for diverse chemical synthesis using two-dimensional array |
| US6268490B1 (en) | 1997-03-07 | 2001-07-31 | Takeshi Imanishi | Bicyclonucleoside and oligonucleotide analogues |
| US6376248B1 (en) | 1997-03-14 | 2002-04-23 | Life Technologies, Inc. | Peptide-enhanced transfections |
| US6670461B1 (en) | 1997-09-12 | 2003-12-30 | Exiqon A/S | Oligonucleotide analogues |
| US6794499B2 (en) | 1997-09-12 | 2004-09-21 | Exiqon A/S | Oligonucleotide analogues |
| WO1999031262A2 (en) | 1997-12-16 | 1999-06-24 | Valentis, Inc. | Needle-free injection of formulated nucleic acid molecules |
| WO1999032619A1 (en) | 1997-12-23 | 1999-07-01 | The Carnegie Institution Of Washington | Genetic inhibition by double-stranded rna |
| US6835393B2 (en) | 1998-01-05 | 2004-12-28 | University Of Washington | Enhanced transport using membrane disruptive agents |
| US6426086B1 (en) | 1998-02-03 | 2002-07-30 | The Regents Of The University Of California | pH-sensitive, serum-stable liposomes |
| WO1999054459A2 (en) | 1998-04-20 | 1999-10-28 | Ribozyme Pharmaceuticals, Inc. | Nucleic acid molecules with novel chemical compositions capable of modulating gene expression |
| US20040132161A1 (en) | 1998-04-20 | 2004-07-08 | Finkel Terri H. | Methods and compositions for increasing CD4lymphocyte immune responsiveness |
| WO2000053722A2 (en) | 1999-03-10 | 2000-09-14 | Phogen Limited | Delivery of nucleic acids and proteins to cells |
| US6849272B1 (en) | 1999-04-21 | 2005-02-01 | Massachusetts Institute Of Technology | Endosomolytic agents and cell delivery systems |
| US7138382B2 (en) | 1999-06-07 | 2006-11-21 | Mirus Bio Corporation | Compositions and methods for drug delivery using pH sensitive molecules |
| WO2001005374A1 (en) | 1999-07-15 | 2001-01-25 | Inex Pharmaceuticals Corp. | Methods for preparation of lipid-encapsulated therapeutic agents |
| US6200599B1 (en) | 1999-10-07 | 2001-03-13 | The Regents Of The University Of California | Ortho ester lipids |
| US20080055370A1 (en) | 2000-03-27 | 2008-03-06 | Fuji Photo Film Co., Ltd. | Multi-nozzle ink jet head and manufacturing method thereof |
| US6843942B2 (en) | 2000-11-03 | 2005-01-18 | Polymun Scientific Immunobilogische Forschung Gmbh | Method and device for producing lipid vesicles |
| US7098032B2 (en) | 2001-01-02 | 2006-08-29 | Mirus Bio Corporation | Compositions and methods for drug delivery using pH sensitive molecules |
| US6897196B1 (en) | 2001-02-07 | 2005-05-24 | The Regents Of The University Of California | pH sensitive lipids based on ortho ester linkers, composition and method |
| US20070252295A1 (en) | 2001-02-21 | 2007-11-01 | Steffen Panzner | Amphoteric liposomes |
| US7108863B2 (en) | 2001-03-26 | 2006-09-19 | Alza Corporation | Liposome composition for improved intracellular delivery of a therapeutic agent |
| US20080055360A1 (en) | 2001-03-27 | 2008-03-06 | Silverbrook Research Pty Ltd | Printhead assembly of printhead integrated circuit modules |
| US20040110296A1 (en) | 2001-05-18 | 2004-06-10 | Ribozyme Pharmaceuticals, Inc. | Conjugates and compositions for cellular delivery |
| US20030130186A1 (en) | 2001-07-20 | 2003-07-10 | Chandra Vargeese | Conjugates and compositions for cellular delivery |
| US20040142025A1 (en) | 2002-06-28 | 2004-07-22 | Protiva Biotherapeutics Ltd. | Liposomal apparatus and manufacturing methods |
| US20080055365A1 (en) | 2002-11-23 | 2008-03-06 | Silverbrook Research Pty Ltd | Inkjet printhead with suspended heater elements |
| US20050064595A1 (en) | 2003-07-16 | 2005-03-24 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering RNA |
| US20060142230A1 (en) | 2003-08-25 | 2006-06-29 | Nastech Pharmaceutical Company Inc. | Double-stranded ribonucleic acid molecules having ribothymidine |
| US20060014289A1 (en) | 2004-04-20 | 2006-01-19 | Nastech Pharmaceutical Company Inc. | Methods and compositions for enhancing delivery of double-stranded RNA or a double-stranded hybrid nucleic acid to regulate gene expression in mammalian cells |
| US20060040882A1 (en) | 2004-05-04 | 2006-02-23 | Lishan Chen | Compostions and methods for enhancing delivery of nucleic acids into cells and for modifying expression of target genes in cells |
| US20080055378A1 (en) | 2004-09-18 | 2008-03-06 | Drury Paul R | Fluid Supply Method and Apparatus |
| US20080055383A1 (en) | 2004-10-07 | 2008-03-06 | Poncelet Olivier J | Inkjet Recording Element |
| US20080055366A1 (en) | 2005-10-31 | 2008-03-06 | Trudy Benjamin | Fluid ejection device |
| US20080055386A1 (en) | 2006-08-29 | 2008-03-06 | Samsung Electronics Co., Ltd. | Medium supplying apparatus of image forming device |
| US20080055339A1 (en) | 2006-08-30 | 2008-03-06 | Marketech International Corp. | Method for automatically detecting and adjusting grayscale/white balance of display |
| US20080055357A1 (en) | 2006-08-31 | 2008-03-06 | Canon Kabushiki Kaisha | Inkjet printing apparatus |
| US20080055385A1 (en) | 2006-09-04 | 2008-03-06 | Fujifilm Corporation | Ink Set and Image Forming Apparatus and Method |
| US20080064417A1 (en) | 2006-09-11 | 2008-03-13 | Research In Motion Limited | Apparatus, and associated method, for paging an access terminal in a radio communication system |
| US20080317839A1 (en) | 2007-05-04 | 2008-12-25 | Nastech Pharmaceutical Company Inc. | Amino acid lipids and uses thereof |
Non-Patent Citations (97)
| Title |
|---|
| A.R. GENNARO: "Remington's Pharmaceutical Sciences", 1985, MACK PUBLISHING CO. |
| AKHTAR ET AL., TRENDS CELL BIO., vol. 2, 1992, pages 139 |
| AKHTAR,: "Delivery Strategies for Antisense Oligonucleotide Therapeutics", 1995 |
| ALDRIAN-HERRADA ET AL., NUCLEIC ACIDS RES., vol. 26, 1998, pages 4910 |
| ALTSCHUL ET AL., J. MOL. BIOL., vol. 215, 1990, pages 403 - 410 |
| BAENZIGER, FIETE, CELL, vol. 22, 1980, pages 611 |
| BAHRAMIAN, ZARBL, MOL. CELL. BIOL., vol. 19, 1999, pages 274 - 283 |
| BEIGELMAN ET AL., J. BIOT CHEM., vol. 270, 1995, pages 25702 |
| BELLON ET AL., BIOCONJUGATE CHEM., vol. 8, 1997, pages 204 |
| BELLON ET AL., NUCLEOSIDES & NUCLEOTIDES, vol. 16, 1997, pages 951 |
| BERGE ET AL., J. PHARM. SCI., vol. 66, 1977, pages 1 9 |
| BERSTEIN ET AL., NATURE, vol. 409, 2001, pages 363 |
| BIOCHEMISTRY, vol. 32, 1993, pages 1751 |
| BOADO ET AL., J. PHARM. SCI., vol. 87, 1998, pages 1308 |
| BOADO, ADV. DRUG DELIVERY REV., vol. 15, 1995, pages 73 |
| BRENNAN ET AL., BIOTECHNOL BIOENG., vol. 61, 1998, pages 33 - 45 |
| BRODY, GOLD, J. BIOTECHNOL., vol. 74, 2000, pages 5 |
| BURGIN ET AL., BIOCHEMISTRY, vol. 35, 1996, pages 14090 |
| BURLINA ET AL., BIOORG. MED. CHEM., vol. 5, 1997, pages 1999 |
| CARUTHERS ET AL., METHODS IN ENZYMOL., vol. 211, 1992, pages 3 - 19 |
| CLOAD, SCHEPARTZ, J. AM. CHEM. SOC., vol. 113, 1991, pages 6324 |
| CONNOLLY ET AL., J. BIOL. CHEM., vol. 257, 1982, pages 939 |
| CONRY ET AL., CLIN. CANCER RES., vol. 5, 1999, pages 2330 |
| CULLIS, BIOCHIMICA ET BIOPHYSICA ACTA, vol. 1463, 2000, pages 107 - 14 |
| DONALD VOET, JUDITH VOET: "Biochemistry, 3rd Edition", 2005, pages: 383 |
| DORSETT, TUSCHL, NATURE REV. DRUG DISCOV., vol. 3, 2004, pages 318 |
| DURAND ET AL., NUCLEIC ACIDS RES., vol. 18, 1990, pages 6353 |
| EAMSHAW, GAIT, BIOPOLYMERS, vol. 48, 1998, pages 39 - 55 |
| ELBASHIR ET AL., EMBO J., vol. 20, 2001, pages 6877 |
| ELBASHIR ET AL., GENES DEV., vol. 15, 2001, pages 188 |
| ELBASHIR ET AL., NATURE, vol. 411, 2001, pages 494 |
| FASMAN: "CRC Practical Handbook of Biochemistry and Molecular Biology", 1989, CRC PRESS, INC. |
| FASMAN: "Practical Handbook of Biochemistry and Molecular Biology", 1989, CRC PRESS, pages: 385 - 394 |
| FERENTZ, VERDINE, J. AM. CHEM. SOC., vol. 113, 1991, pages 4000 |
| FIRE ET AL., NATURE, vol. 391, 1998, pages 806 |
| FRIER ET AL., PROC. NAT'L. ACAD. SCI. USA, vol. 83, 1986, pages 9373 |
| G. GREGORIADIS: "Liposome Technology", 1984, CRC PRESS |
| GOLD ET AL., ANNU. REV. BIOCHEM., vol. 64, 1995, pages 763 |
| GREG T. HERMANSON, BIOCONJUGATE TECHNIQUES, 1996 |
| HAMILTON ET AL., SCIENCE, vol. 286, 1999, pages 950 - 951 |
| HERDEWIJN, ANTISENSE NUCLEIC ACID DRUG DEV., vol. 10, 2000, pages 297 |
| HERMANN, PATEL, SCIENCE, vol. 287, 2000, pages 820 |
| HOFLAND, HUANG, HANDB. EXP. PHARMACOL, vol. 137, 1999, pages 165 |
| HUNZIKER, LEUMANN: "Nucleic Acid Analogues: Synthesis and Properties, in Modem Synthetic Methods", 1995, VCH, pages: 331 - 417 |
| ISHIWATA ET AL., CHEM. PHARM. BULL., vol. 43, 1995, pages 1005 |
| JASCHKE ET AL., TETRAHEDRON LETT., vol. 34, 1993, pages 301 |
| JAYASENA, CLINICAL CHEM., vol. 45, 1999, pages 1628 |
| KARPEISKY ET AL., TETRAHEDRON LETT., vol. 39, 1998, pages 1131 |
| KIM ET AL., NATURE BIOTECH., vol. 23, 2005, pages 222 |
| KURRECK, EUR. J. BIOCHEM., vol. 270, 2003, pages 1628 |
| KUSSER, J. BIOTECHNOL., vol. 74, 2000, pages 27 |
| LASIC ET AL., CHEM. REV., vol. 95, 1995, pages 2601 |
| LASIC ET AL., SCIENCE, vol. 267, 1995, pages 1275 |
| LEE ET AL., ACS SYMP. SER., vol. 752, 2000, pages 184 |
| LEE, LEE, GLYCOCONJUGATE J., vol. 4, 1987, pages 317 |
| LEROY G. WADE, COMPENDIUM OF ORGANIC SYNTHETIC METHODS, 1980 |
| LEUSCHNER ET AL., EMBO, vol. 7, 2006, pages 314 |
| LIU ET AL., J. BIOL. CHEM., vol. 42, 1995, pages 24864 |
| LOAKES ET AL., J. MOL. BIO., vol. 270, 1997, pages 426 |
| LOAKES, NUCLEICACIDS RES., vol. 29, 2001, pages 2437 |
| M. J. OSTRO: "Liposomes", 1987, MARCEL DEKKER |
| MA ET AL., NUCLEIC ACIDS RES., vol. 21, 1993, pages 2585 |
| MAURER ET AL., MOL. MEMBR. BIOL., vol. 16, 1999, pages 129 |
| MCCURDY ET AL., NUCLEOSIDES & NUCLEOTIDES, vol. 10, 1991, pages 287 |
| MESMAEKER ET AL., ACS, 1994, pages 24 - 39 |
| MICHAEL B. SMITH, JERRY MARCH: "March's Advanced Organic Chemistry, 5th edition,", 2001 |
| MOORE ET AL., SCIENCE, vol. 256, 1992, pages 9923 |
| NUCLEIC ACIDS RES., vol. 15, 1987, pages 3113 |
| OKU ET AL., BIOCHIM. BIOPHYS. ACTA, vol. 1238, 1995, pages 86 |
| ONO ET AL., BIOCHEMISTRY, vol. 30, 1991, pages 9914 |
| P. HEINRICH STAHL: "Handbook of Pharmaceutical Salts", 2002 |
| PARDRIDGE ET AL., PROC. NAT'L ACAD. SCI. USA, vol. 92, 1995, pages 5592 |
| PERRAULT ET AL., NATURE, vol. 344, 1990, pages 565 |
| PIEKEN ET AL., SCIENCE, vol. 253, 1991, pages 314 |
| PONPIPOM ET AL., J. MED. CHEM., vol. 24, 1981, pages 1388 |
| R. L. DUNN, R. M. OTTENBRITE: "Polymeric Drugs and Drug Delivery Systems", 1991, ACS SYMP. SER., pages: 469 |
| RICHARDSON, SCHEPARTZ, J. AM. CHEM. SOC., vol. 113, 1991, pages 5109 |
| SCARINGE ET AL., NUCLEIC ACIDS RES., vol. 18, 1990, pages 5433 |
| SEELA, KAISER, NUCLEIC ACIDS RES., vol. 18, 1990, pages 6353 |
| SHABAROVA ET AL., NUCLEIC ACIDS RES., vol. 19, 1991, pages 4247 |
| STANLEY R. SANDLER, WOLF KARO, ORGANIC FUNCTIONAL GROUP PREPARATIONS, 1989 |
| SUN, CURR. OPIN. MOL. THER., vol. 2, 2000, pages 100 |
| T. W. GREENE, P. G. M. WUTS: "Protective Groups In Organic Synthesis(3rd ed.", 1991 |
| TURNER ET AL., CSH SYMP. TUANT. BIOL. LLL, 1987, pages 123 |
| TURNER ET AL., J. AM. CHEM. SOC., vol. 109, 1987, pages 3783 |
| TYLER ET AL., FEBS LETT., vol. 421, 1999, pages 280 |
| TYLER ET AL., PROC. NAT'L ACAD. SCI. USA, vol. 96, 1999, pages 7053 - 7058 |
| USMAN ET AL., J. AM. CHEM. SOC., vol. 109, 1987, pages 7845 |
| USMAN, CEDERGREN, TRENDS IN BIOCHEM. SCI., vol. 17, 1992, pages 334 |
| USMAN, NUCLEIC ACIDS SYMP. SER., vol. 31, 1994, pages 163 |
| VERMA, ECKSTEIN, ANNU. REV. BIOCHEM., vol. 67, 1998, pages 99 - 134 |
| VOCERO-AKBANI ET AL., NATURE MED., vol. 5, 1999, pages 23 |
| WIANNY, GOETZ, NATURE CELL BIOL., vol. 2, 1999, pages 70 |
| WINCOTT ET AL., METHODS MOL. BIO., vol. 74, 1997, pages 59 |
| WINCOTT ET AL., NUCLEIC ACIDS RES., vol. 23, 1995, pages 2677 - 2684 |
| WU, WU, J. BIOL. CHEM., vol. 262, 1987, pages 4429 |
| Z. H. HUANG, F. C. SZOKA: "Nanoparticle Technology, 3rd ed.", 2006, CRC PRESS, article "Bioresponsive nanoparticles and their use for macromolecular delivery" |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017510583A (en) * | 2014-03-25 | 2017-04-13 | アークトゥラス・セラピューティクス・インコーポレイテッドArcturus Therapeutics,Inc. | Transthyretin allele-selective UNA oligomers for gene silencing |
| EP3122889A4 (en) * | 2014-03-25 | 2017-11-01 | Arcturus Therapeutics, Inc. | Una oligomers having reduced off-target effects in gene silencing |
| US10604758B2 (en) | 2014-03-25 | 2020-03-31 | Arcturus Therapeutics, Inc. | Therapeutic oligomers for treating amyloidosis |
| US10683500B2 (en) | 2014-03-25 | 2020-06-16 | Arcturus Therapeutics, Inc. | UNA oligomers having reduced off-target effects in gene silencing |
| EP3122365B1 (en) * | 2014-03-25 | 2023-05-03 | Arcturus Therapeutics, Inc. | Transthyretin allele selective una oligomers for gene silencing |
| US10519447B2 (en) | 2015-04-01 | 2019-12-31 | Arcturus Therapeutics, Inc. | Therapeutic UNA oligomers and uses thereof |
| US10421964B2 (en) | 2015-07-23 | 2019-09-24 | Arcturus Therapeutics, Inc. | UNA oligomers and compositions for treating amyloidosis |
| WO2020016894A1 (en) * | 2018-07-19 | 2020-01-23 | Yeda Research And Development Co. Ltd. | Sphingosine analogs and use thereof against bacterial lung infections |
| US11471430B2 (en) | 2018-07-19 | 2022-10-18 | Yeda Research And Development Co. Ltd. | Sphingosine analogs and use thereof against bacterial lung infections |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2011133584A3 (en) | 2012-01-12 |
| WO2011133584A8 (en) | 2012-06-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6126072B2 (en) | Processes and compositions for efficient delivery by liposomes in therapy to suppress gene expression | |
| JP5864632B2 (en) | Amino acid lipids and uses thereof | |
| AU2008342535B2 (en) | Silencing of polo-like kinase expression using interfering RNA | |
| CN104922676B (en) | Polyamine derivatives | |
| CN103987847B (en) | Amine cation lipid and application thereof | |
| WO2009046220A2 (en) | Lipopeptides for delivery of nucleic acids | |
| WO2011133584A2 (en) | Nucleic acid compounds for inhibiting hras gene expression and uses thereof | |
| WO2011139842A2 (en) | Nucleic acid compounds for inhibiting fgfr3 gene expression and uses thereof | |
| AU2014202674B2 (en) | Amino acid lipids and uses thereof | |
| WO2011120023A1 (en) | Nucleic acid compounds for inhibiting survivin gene expression uses thereof | |
| AU2014262213B2 (en) | Amino acid lipids and uses thereof | |
| AU2013202970A1 (en) | Silencing of polo-like kinase expression using interfering RNA |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11719919 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11719919 Country of ref document: EP Kind code of ref document: A2 |