Analysis of the Physico-Chemical, Mechanical and Biological Properties of Crosslinked Type-I Collagen from Horse Tendon: Towards the Development of Ideal Scaffolding Material for Urethral Regeneration
Abstract
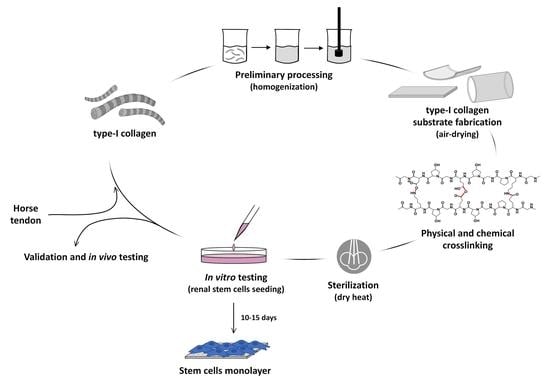
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collagen Substrates Preparation
2.3. Composition
2.4. Crosslinking Degree
2.5. Secondary Structure
2.6. Swelling Degree
2.7. Thermal Stability
2.8. Degradation Resistance
2.9. Surface Properties
2.10. Mechanical Properties
2.11. In Vitro Response: Cell Culture and Immunostaining
2.12. Statistical Analysis
3. Results
3.1. Composition/Purity
3.2. Crosslinking Degree
3.3. Secondary Structure
3.4. Swelling Degree
3.5. Thermal Properties
3.6. Degradation Resistance
3.7. Surface Properties
3.8. Mechanical Properties
3.9. Biological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male Urethral Stricture Disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- NHS England. Urology, Specialised Services Clinical Reference Group for Specialised Urology, Clinical Commissioning Policy: Urethroplasty for Benign Urethral Strictures in Adult Men. Available online: https://www.england.nhs.uk/wp-content/uploads/2018/07/Urethroplasty-for-benign-urethral-strictures-in-adult-men.pdf (accessed on 4 December 2020).
- Watkin, N.; Patel, P. The Diagnosis and Management of Acquired Urethral Stricture Disease. Surgery 2017, 35, 313–323. [Google Scholar] [CrossRef]
- Cheng, L.; Li, S.; Wang, Z.; Huang, B.; Lin, J. A Brief Review on Anterior Urethral Strictures. Asian J. Urol. 2018, 5, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Nuss, G.R.; Granieri, M.A.; Zhao, L.C.; Thum, D.J.; Gonzalez, C.M. Presenting Symptoms of Anterior Urethral Stricture Disease: A Disease Specific, Patient Reported Questionnaire to Measure Outcomes. J. Urol. 2012, 187, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Rourke, K.; Hickle, J. The Clinical Spectrum of the Presenting Signs and Symptoms of Anterior Urethral Stricture: Detailed Analysis of a Single Institutional Cohort. Urology 2012, 79, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Geist, E.; Hartung, R. In the Treatment of Urethral Strictures—Review of the Current Literature. In Urethral Reconstructive Surgery; Schreiter, F., Jordan, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Benson, C.R.; Goldfarb, R.; Kirk, P.; Qin, Y.; Borza, T.; Skolarus, T.A.; Brandes, S.B. Population Analysis of Male Urethral Stricture Management and Urethroplasty Success in the United States. Urology 2019, 123, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.M.; Venkatesan, K. Endoscopic Management of Urethral Stricture: Review and Practice Algorithm for Management of Male Urethral Stricture Disease. Curr. Urol. Rep. 2018, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Verla, W.; Oosterlinck, W.; Spinoit, A.F.; Waterloos, M.; Martins, F.E. A Comprehensive Review Emphasizing Anatomy, Etiology, Diagnosis, and Treatment of Male Urethral Stricture Disease. Biomed Res. Int. 2019, 2019, 9046430. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.E.; Simoes de Oliveira, P.; Martins, N.M. Historical Perspective and Innovations in Penile Urethroplasty. In Lower Urinary Tract Dysfunction—From Evidence to Clinical Practice; IntechOpen: London, UK, 2019; pp. 1–35. [Google Scholar] [CrossRef] [Green Version]
- Selim, M.; Salem, S.; Elsherif, E.; Badawy, A.; Elshazely, M.; Gawish, M. Outcome of Staged Buccal Mucosal Graft for Repair of Long Segment Anterior Urethral Stricture. BMC Urol. 2019, 19, 38. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, A. Substitution Urethroplasty Using Oral Mucosa Graft for Male Anterior Urethral Stricture Disease: Current Topics and Reviews. Int. J. Urol. 2017, 24, 493–503. [Google Scholar] [CrossRef]
- Wood, D.N.; Allen, S.E.; Andrich, D.E.; Greenwell, T.J.; Mundy, A.R. The Morbidity of Buccal Mucosal Graft Harvest for Urethroplasty and the Effect of Nonclosure of the Graft Harvest Site on Postoperative Pain. J. Urol. 2004, 172, 580–583. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Barbagli, G.; Sansalone, S.; Joshi, P.M. Harvesting Oral Mucosa for One-Stage Anterior Urethroplasty. Indian J. Urol. 2014, 30, 117–121. [Google Scholar] [CrossRef]
- Hillary, C.J.; Osman, N.I.; Chapple, C.R. Current Trends in Urethral Stricture Management. Asian J. Urol. 2014, 1, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Q.; Fu, Q. Tissue Engineering for Urinary Tract Reconstruction and Repair: Progress and Prospect in China. Asian J. Urol. 2018, 5, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.G.; Li, Z.; Chen, S.Y.; Xie, M.K.; Huang, J.W.; Peng, X.F.; Yang, R.X.; Wang, H.P.; Xu, Y.M.; Feng, C. Structural and Functional Evaluation of Oxygenating Keratin/Silk Fibroin Scaffold and Initial Assessment of Their Potential for Urethral Tissue Engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef]
- Jia, W.; Tang, H.; Wu, J.; Hou, X.; Chen, B.; Chen, W.; Zhao, Y.; Shi, C.; Zhou, F.; Yu, W.; et al. Urethral Tissue Regeneration Using Collagen Scaffold Modified with Collagen Binding VEGF in a Beagle Model. Biomaterials 2015, 69, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered Acellular Collagen Scaffold for Endogenous Cell Guidance, a Novel Approach in Urethral Regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Vacanti, C.A. The History of Tissue Engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Tampieri, A.; Salvatore, L.; Sannino, A.; Ghiron, J.H.L.; Condorelli, G. Collagen Based Scaffold for Biomedical Applications. J. Biotechnol. 2010, 150, 29. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef]
- Gallo, N.; Nasser, H.; Salvatore, L.; Natali, M.L.; Campa, L.; Mahmoud, M.; Capobianco, L.; Sannino, A.; Madaghiele, M. Hyaluronic Acid for Advanced Therapies: Promises and Challenges. Eur. Polym. J. 2019, 117, 134–147. [Google Scholar] [CrossRef]
- Daamen, W.F.; Veerkamp, J.H.; van Hest, J.C.M.; van Kuppevelt, T.H. Elastin as a Biomaterial for Tissue Engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Tang, F.; Whitelock, J.M.; Lord, M.S. Glycosaminoglycan and Proteoglycan-Based Biomaterials: Current Trends and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 1701042. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [Green Version]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Avila Rodriguez, M.I.; Rodriguez Barroso, G.L.; Sanchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An Insight on Type I Collagen from Horse Tendon for the Manufacture of Implantable Devices. Int. J. Biol. Macromol. 2020, 154, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and Immunogenicity of Collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Ellingsworth, L.R.; De Lustro, F.; Brennan, J.E.; Sawamura, S.; Mc Pherson, J. The Human Immune Response to Reconstituted Bovine Collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar] [PubMed]
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a Bovine Collagen Implant: Clinical and Immunologic Study in 705 Patients. J. Am. Acad. Dermatol. 1989, 21, 1203–1208. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Sparavigna, A.; Tateo, A.; Inselvini, E.; Tocchio, M.; Orlandini, M.C.; Botali, G. Anti-Age Activity and Tolerance Evaluation of Collagen Micro-Injection Treatment Associated to Topical Application of a Cosmetic Formulation (Investigator-Initiated Multicentre Trial). J. Clin. Exp. Dermatol. Res. 2017, 8, 1000391. [Google Scholar] [CrossRef]
- Bianchini, P.; Parma, B. Immunological Safety Evaluation of a Horse Collagen Haemostatic Pad. Arzneimittelforschung 2001, 51, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Adelmann-Grill, B.C.; Otto, K. Immunological Safety Evaluation of a Haemostatic Agent and Wound Dressing Made of Horse Collagen Fibrils. Arzneimittelforschung 1987, 37, 802–805. [Google Scholar]
- Abdal-Hameed, I.A. Clinical and Radiographical Assessment of Topical Application of Collagen Fibrils on Tooth Socket Healing. Al-Rafidain Dent. J. 2014, 14, 244–251. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of Different Collagen Species on Physico-Chemical Properties of Crosslinked Collagen Matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Steele, K.E.; Twenhafel, N.A. Review Paper: Pathology of Animal Models of Alphavirus Encephalitis. Vet. Pathol. 2010, 47, 790–805. [Google Scholar] [CrossRef]
- Wong, K.T. Alphaviral Equine Encephalomyelitis (Eastern, Western, and Venezuelan). In Infections of the Central Nervous System: Pathology and Genetics; Wiley: Hoboken, NJ, USA, 2020; pp. 183–187. [Google Scholar]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of Processing on Structural, Mechanical and Biological Properties of Collagen-Based Substrates for Regenerative Medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laghezza Masci, V.; Taddei, A.R.; Gambellini, G.; Giorgi, F.; Fausto, A.M. Ultrastructural Investigation on Fibroblast Interaction with Collagen Scaffold. J. Biomed. Mater. Res. Part A 2016, 104, 272–282. [Google Scholar] [CrossRef]
- Croce, M.A.; Silvestri, C.; Guerra, D.; Carnevali, E.; Boraldi, F.; Tiozzo, R.; Parma, B. Adhesion and Proliferation of Human Dermal Fibroblasts on Collagen Matrix. J. Biomater. Appl. 2004, 18, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Strauß, C.; Stoiber, S.; Kasper, C.; Charwat, V. Impact of Source and Manufacturing of Collagen Matrices on Fibroblast Cell Growth and Platelet Aggregation. Materials 2017, 10, 1086. [Google Scholar] [CrossRef] [Green Version]
- Giampetruzzi, L.; Blasi, L.; Quarta, A.; Argentiere, S.; Cella, C.; Salvatore, L.; Madaghiele, M.; Gigli, G.; Sannino, A. Poly(Lactide-Co-Glycolide) Nanoparticles Embedded in a Micropatterned Collagen Scaffold for Neuronal Tissue Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 359–368. [Google Scholar] [CrossRef]
- Madaghiele, M.; Calò, E.; Salvatore, L.; Bonfrate, V.; Pedone, D.; Frigione, M.; Sannino, A. Assessment of Collagen Crosslinking and Denaturation for the Design of Regenerative Scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Piccinno, A.; Saponaro, M.; Maffezzoli, A.; Sannino, A. Collagen- and Gelatine-Based Films Sealing Vascular Prostheses: Evaluation of the Degree of Crosslinking for Optimal Blood Impermeability. J. Mater. Sci. Mater. Med. 2009, 20, 1979–1989. [Google Scholar] [CrossRef]
- Salvatore, L.; Madaghiele, M.; Parisi, C.; Gatti, F.; Sannino, A. Crosslinking of Micropatterned Collagen-Based Nerve Guides to Modulate the Expected Half-Life. J. Biomed. Mater. Res. Part A 2014, 102, 4406–4414. [Google Scholar] [CrossRef]
- Parisi, C.; Salvatore, L.; Veschini, L.; Serra, M.P.; Hobbs, C.; Madaghiele, M.; Sannino, A.; Di Silvio, L. Biomimetic Gradient Scaffold of Collagen–Hydroxyapatite for Osteochondral Regeneration. J. Tissue Eng. 2020, 11, 2041731419896068. [Google Scholar] [CrossRef] [Green Version]
- Monaco, G.; Cholas, R.; Salvatore, L.; Madaghiele, M.; Sannino, A. Sterilization of Collagen Scaffolds Designed for Peripheral Nerve Regeneration: Effect on Microstructure, Degradation and Cellular Colonization. Mater. Sci. Eng. C 2017, 71, 335–344. [Google Scholar] [CrossRef]
- Gallo, N.; Lunetti, P.; Bettini, S.; Barca, A.; Madaghiele, M.; Valli, L.; Capobianco, L.; Sannino, A.; Salvatore, L. Assessment of Physico-Chemical and Biological Properties of Sericin-Collagen Substrates for PNS Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 403–413. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S.; Foresti, E.; Parma, B.; Rubini, K.; Sidoti, M.C.; Roveri, N. Films of Self-Assembled Purely Helical Type I Collagen Molecules. J. Mater. Chem. 2004, 14, 2297–2302. [Google Scholar] [CrossRef]
- Ruozi, B.; Tosi, G.; Leo, E.; Parma, B.; Vismara, S.; Forni, F.; Vandelli, M.A. Intact Collagen and Atelocollagen Sponges: Characterization and ESEM Observation. Mater. Sci. Eng. C 2007, 27, 802–810. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Campa, L.; Natali, M.L.; Salvatore, L.; Madaghiele, M.; De Caro, L.; et al. Investigations of Processing–Induced Structural Changes in Horse Type-i Collagen at Sub and Supramolecular Levels. Front. Bioeng. Biotechnol. 2019, 7, 203. [Google Scholar] [CrossRef]
- Lawrence, A.M.; Besir, H. Staining of Proteins in Gels with Coomassie G-250 without Organic Solvent and Acetic Acid. J. Vis. Exp. 2009, 30, 1350. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of Cell Binding to Collagen and Gelatin: A Study of the Effect of 2D and 3D Architecture and Surface Chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofner, C.M.; Bubnis, W.A. Chemical and Swelling Evaluations of Amino Group Crosslinking in Gelatin and Modified Gelatin Matrices. Pharm. Res. 1996, 13, 1821–1827. [Google Scholar] [CrossRef]
- Kale, R.N.; Bajaj, A.N. Ultraviolet Spectrophotometric Method for Determination of Gelatin Crosslinking in the Presence of Amino Groups. J. Young Pharm. 2010, 2, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Li, B.; Jiang, D.; Hou, H. Nile Tilapia Skin Collagen Sponge Modified with Chemical Cross-Linkers as a Biomedical Hemostatic Material. Colloids Surf. B Biointerfaces 2017, 159, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Snider, S.; Cavalli, A.; Colombo, F.; Gallotti, A.L.; Quattrini, A.; Salvatore, L.; Madaghiele, M.; Terreni, M.R.; Sannino, A.; Mortini, P. A Novel Composite Type I Collagen Scaffold with Micropatterned Porosity Regulates the Entrance of Phagocytes in a Severe Model of Spinal Cord Injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1040–1053. [Google Scholar] [CrossRef]
- Bettini, S.; Bonfrate, V.; Syrgiannis, Z.; Sannino, A.; Salvatore, L.; Madaghiele, M.; Valli, L.; Giancane, G. Biocompatible Collagen Paramagnetic Scaffold for Controlled Drug Release. Biomacromolecules 2015, 16, 2599–2608. [Google Scholar] [CrossRef]
- Miles, C.A.; Avery, N.C.; Rodin, V.V.; Bailey, A.J. The Increase in Denaturation Temperature Following Cross-Linking of Collagen Is Caused by Dehydration of the Fibres. J. Mol. Biol. 2005, 346, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Kodre, K.; Attarde, S.; Yendhe, P.; Patil, R.; Barge, V. Research and Reviews: Journal of Pharmaceutical Analysis. Res. Rev. J. Pharm. Anal. Differ. 2014, 3, 11–22. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Salvatore, L.; Carofiglio, V.E.; Stufano, P.; Bonfrate, V.; Calò, E.; Scarlino, S.; Nitti, P.; Centrone, D.; Cascione, M.; Leporatti, S.; et al. Potential of Electrospun Poly(3-Hydroxybutyrate)/Collagen Blends for Tissue Engineering Applications. J. Healthc. Eng. 2018, 2018, 6573947. [Google Scholar] [CrossRef] [PubMed]
- Nitti, P.; Gallo, N.; Natta, L.; Scalera, F.; Palazzo, B.; Sannino, A.; Gervaso, F. Influence of Nanofiber Orientation on Morphological and Mechanical Properties of Electrospun Chitosan Mats. J. Healthc. Eng. 2018, 2018, 3651480. [Google Scholar] [CrossRef] [PubMed]
- Catto, V.; Farè, S.; Cattaneo, I.; Figliuzzi, M.; Alessandrino, A.; Freddi, G.; Remuzzi, A.; Tanzi, M.C. Small Diameter Electrospun Silk Fibroin Vascular Grafts: Mechanical Properties, in Vitro Biodegradability, and in Vivo Biocompatibility. Mater. Sci. Eng. C 2015, 54, 101–111. [Google Scholar] [CrossRef]
- Johnson, J.; Ohst, D.; Groehl, T.; Hetterscheidt, S.; Jones, M. Development of Novel, Bioresorbable, Small-Diameter Electrospun Vascular Grafts. J. Tissue Sci. Eng. 2015, 6, 2. [Google Scholar] [CrossRef]
- Sallustio, F.; Curci, C.; Aloisi, A.; Toma, C.C.; Marulli, E.; Serino, G.; Cox, S.N.; De Palma, G.; Stasi, A.; DIvella, C.; et al. Inhibin-A and Decorin Secreted by Human Adult Renal Stem/Progenitor Cells Through the TLR2 Engagement Induce Renal Tubular Cell Regeneration. Sci. Rep. 2017, 7, 8225. [Google Scholar] [CrossRef] [Green Version]
- Sallustio, F.; Serino, G.; Costantino, V.; Curci, C.; Cox, S.N.; De Palma, G.; Schena, F.P. MiR-1915 and MiR-1225-5p Regulate the Expression of CD133, PAX2 and TLR2 in Adult Renal Progenitor Cells. PLoS ONE 2013, 8, e68296. [Google Scholar] [CrossRef]
- Sallustio, F.; De Benedictis, L.; Castellano, G.; Zaza, G.; Loverre, A.; Costantino, V.; Grandaliano, G.; Schena, F.P. TLR2 Plays a Role in the Activation of Human Resident Renal Stem/Progenitor Cells. FASEB J. 2010, 24, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, F.; Stasi, A.; Curci, C.; Divella, C.; Picerno, A.; Franzin, R.; de Palma, G.; Rutigliano, M.; Lucarelli, G.; Battaglia, M.; et al. Renal Progenitor Cells Revert LPS-Induced Endothelial-to-Mesenchymal Transition by Secreting CXCL6, SAA4, and BPIFA2 Antiseptic Peptides. FASEB J. 2019, 33, 10753–10766. [Google Scholar] [CrossRef] [PubMed]
- Gorham, S.D.; Light, N.D.; Diamond, A.M.; Willins, M.J.; Bailey, A.J.; Wess, T.J.; Leslie, N.J. Effect of Chemical Modifications on the Susceptibility of Collagen to Proteolysis. II. Dehydrothermal Crosslinking. Int. J. Biol. Macromol. 1992, 14, 129–138. [Google Scholar] [CrossRef]
- Lu, M.; Song, X.; Yang, M.; Kong, W.; Zhu, J. Combined Effects of Glutaraldehyde and Riboflavin/Uv365 on the Self-Assembly of Type I Collagen Molecules Observed with Atomic Force Microscopy. Int. J. Food Prop. 2018, 21, 2181–2192. [Google Scholar] [CrossRef] [Green Version]
- US Pharmacopeia Inc. USP Monographs: Small Intestinal Submucosa Wound Matrix. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0m541.html (accessed on 2 February 2020).
- Hurst, R.E.; Bonner, R.B. Mapping of the Distribution of Significant Proteins and Proteoglycans in Small Intestinal Submucosa by Fluorescence Microscopy. J. Biomater. Sci. Polym. Ed. 2001, 12, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X.; Chao, N.-N.; Qin, T.-W.; Ding, W.; Zhang, Y.; Sang, J.-W.; Luo, J.-C. Preparation and Characterization of Pro-Angiogenic Gel Derived from Small Intestinal Submucosa. Acta Biomater. 2016, 29, 135–148. [Google Scholar] [CrossRef]
- Hodde, J.P.; Badylak, S.F.; Brightman, A.O.; Voytik-Harbin, S.L. Glycosaminoglycan Content of Small Intestinal Submucosa: A Bioscaffold for Tissue Replacement. Tissue Eng. 1996, 2, 209–217. [Google Scholar] [CrossRef]
- McPherson, T.B.; Badylak, S.F. Characterization of Fibronectin Derived from Small Intestinal Submucosa. Tissue Eng. 1998, 4, 75–83. [Google Scholar] [CrossRef]
- Shi, L.; Ronfard, V. Biochemical and Biomechanical Characterization of Porcine Small Intestinal Submucosa (SIS): A Mini Review. Int. J. Burn. Trauma 2013, 3, 173–179. [Google Scholar]
- Arslan, Y.E.; Efe, B.; Sezgin Arslan, T. A Novel Method for Constructing an Acellular 3D Biomatrix from Bovine Spinal Cord for Neural Tissue Engineering Applications. Biotechnol. Prog. 2019, 35, e2814. [Google Scholar] [CrossRef]
- Wu, P.; Kimura, T.; Tadokoro, H.; Nam, K.; Fujisato, T.; Kishida, A. Relation between the Tissue Structure and Protein Permeability of Decellularized Porcine Aorta. Mater. Sci. Eng. C 2014, 43, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.K.; Salvatore, L.; Gervaso, F.; Catalano, M.; Taurino, A.; Sannino, A.; Licciulli, A. Synthesis and Characterization of Collagen Scaffolds Reinforced by Eggshell Derived Hydroxyapatite for Tissue Engineering. J. Nanosci. Nanotechnol. 2015, 15, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.C.; Madaghiele, M.; Salvatore, L.; Barca, A.; Scialla, S.; Bettini, S.; Valli, L.; Verri, T.; Bucalá, V.; Sannino, A. Integration of PLGA Microparticles in Collagen-Based Matrices: Tunable Scaffold Properties and Interaction Between Microparticles and Human Epithelial-Like Cells. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 137–147. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Z.; Qi, F.; Jin, L.; Zhang, L.; Zhu, N. Polyethylene-Glycol-Ornamented Small Submucosa Bio-Sponge for Skin Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 2457–2465. [Google Scholar] [CrossRef]
- Wang, H.M.; Chou, Y.T.; Wen, Z.H.; Wang, Z.R.; Chen, C.H.; Ho, M.L. Novel Biodegradable Porous Scaffold Applied to Skin Regeneration. PLoS ONE 2013, 8, e56330. [Google Scholar] [CrossRef]
- Samouillan, V.; Delaunay, F.; Dandurand, J.; Merbahi, N.; Gardou, J.P.; Yousfi, M.; Gandaglia, A.; Spina, M.; Lacabanne, C. The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. J. Funct. Biomater. 2011, 2, 230–248. [Google Scholar] [CrossRef] [Green Version]
- Pieper, J.S.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Development of Tailor-Made Collagen–Glycosaminoglycan Matrices: EDC/NHS Crosslinking, and Ultrastructural Aspects. Biomaterials 2000, 21, 581–593. [Google Scholar] [CrossRef]
- Danielsen, C.C. Difference in Thermal Stability of Type-I and Type-III Collagen from Rat Skin. Biochem. J. 1982, 203, 323–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzin, G.; Scanola, M.; Gotte, L. The Low-temperature Mechanical Relaxation of Elastin. I. The Dry Protein. Biopolymers 1976, 15, 283–292. [Google Scholar] [CrossRef]
- Bose, S.; Narayan, R.; Bandyopadhyay, A. Biomaterials Science: Processing, Properties and Applications III; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Grover, C.N.; Cameron, R.E.; Best, S.M. Investigating the Morphological, Mechanical and Degradation Properties of Scaffolds Comprising Collagen, Gelatin and Elastin for Use in Soft Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef] [PubMed]
- El-Assmy, A.; El-Hamid, M.A.; Hafez, A.T. Urethral Replacement: A Comparison between Small Intestinal Submucosa Grafts and Spontaneous Regeneration. BJU Int. 2004, 94, 1132–1135. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Liu, B.; Wang, Y.; Chu, J.; Xiong, G.; Shen, L.; Long, C.; Lin, T.; He, D.; et al. Urethral Reconstruction with Autologous Urine-Derived Stem Cells Seeded in Three-Dimensional Porous Small Intestinal Submucosa in a Rabbit Model. Stem Cell Res. Ther. 2017, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Du, A.; Li, J.; Pan, M.; Han, W.; Xiao, Y. Development of a Cell-Seeded Modified Small Intestinal Submucosa for Urethroplasty. Heliyon 2016, 2, e00087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental Insight into the Effect of Carbodiimide Crosslinking on Cellular Recognition of Collagen-Based Scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Wong, S.S.W.; Aboumarzouk, O.M.; Narahari, R.; O’Riordan, A.; Pickard, R. Simple Urethral Dilatation, Endoscopic Urethrotomy, and Urethroplasty for Urethral Stricture Disease in Adult Men. Cochrane Database Syst. Rev. 2012, 12, CD006934. [Google Scholar] [CrossRef] [Green Version]
- Barbagli, G.; Fossati, N.; Sansalone, S.; Larcher, A.; Romano, G.; Dell’Acqua, V.; Guazzoni, G.; Lazzeri, M. Prediction of Early and Late Complications after Oral Mucosal Graft Harvesting: Multivariable Analysis from a Cohort of 553 Consecutive Patients. Urol. J. 2014, 191, 688–693. [Google Scholar] [CrossRef]
- Micol, L.A.; Arenas da Silva, L.F.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.J.; Frey, P. In-Vivo Performance of High-Density Collagen Gel Tubes for Urethral Regeneration in a Rabbit Model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.; Osman, N.; MacNeil, S. Developing Tissue-Engineered Solutions for the Treatment of Extensive Urethral Strictures. Eur. Urol. 2013, 63, 539–541. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Y.M.; Fu, Q.; Zhu, W.D.; Cui, L.; Chen, J. Evaluation of the Biocompatibility and Mechanical Properties of Naturally Derived and Synthetic Scaffolds for Urethral Reconstruction. J. Biomed. Mater. Res. A 2010, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Bessho, M.; Furuta, M.; Kojima, T.; Okuda, S.; Hara, M. A Novel Collagen Hydrogel Crosslinked by Gamma-Ray Irradiation in Acidic PH Conditions. J. Biomater. Sci. Polym. Edn. 2006, 17, 837–858. [Google Scholar] [CrossRef]
- Sallustio, F.; Curci, C.; Stasi, A.; De Palma, G.; Divella, C.; Gramignoli, R.; Castellano, G.; Gallone, A.; Gesualdo, L. Role of Toll-like Receptors in Actuating Stem/Progenitor Cell Repair Mechanisms: Different Functions in Different Cells. Stem Cells Int. 2019, 2019, 6795845. [Google Scholar] [CrossRef] [Green Version]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin Stress Fibers—Assembly, Dynamics and Biological Roles. J. Cell. Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef] [Green Version]
- Zemel, A.; Rehfeldt, F.; Brown, A.E.; Discher, D.E.; Safran, S.A. Optimal Matrix Rigidity for Stress Fiber Polarization in Stem Cells. Nat. Phys. 2010, 6, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Tan, J.; Tien, J. Mechanotransduction at Cell-Matrix and Cell-Cell Contacts. Annu. Rev. Biomed. Eng. 2004, 6, 275–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brossa, A.; Papadimitriou, E.; Collino, F.; Incarnato, D.; Oliviero, S.; Camussi, G.; Bussolati, B. Role of CD133 Molecule in Wnt Response and Renal Repair. Stem Cells Transl. Med. 2018, 7, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Li, Z. CD133: A Stem Cell Biomarker and Beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzhor, E.; Harari-Steinberg, O.; Omer, D.; Metsuyanim, S.; Jacob-Hirsch, J.; Noiman, T.; Dotan, Z.; Goldstein, R.S.; Dekel, B. Kidney Spheroids Recapitulate Tubular Organoids Leading to Enhanced Tubulogenic Potency of Human Kidney-Derived Cells. Tissue Eng. Part. A 2011, 17, 2305–2319. [Google Scholar] [CrossRef] [PubMed]











| Sample | G (kPa) | ρ (mol/cm3) | E (MPa) | σmax (MPa) | εr (%) | SR (g) |
|---|---|---|---|---|---|---|
| DHT | 0.08 ± 0.01 | 4.8 ± 0.3 × 10−4 | 2.9 ± 0.5 | 2.3 ± 1.2 | 55.6 ± 20.7 | 52 ± 7 |
| DHT + EDC | 0.60 ± 0.11 | 17.0 ± 4.1×10−4 | 8.3 ± 1.4 | 3.4 ± 1.0 | 39.1 ± 11.0 | 24 ± 3 |
| SIS | 0.23 ± 0.04 | 7.6 ± 0.9 × 10−4 | 8.8 ± 1.7 | 7.1 ± 0.8 | 40.8 ± 5.9 | 520 ± 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, N.; Natali, M.L.; Curci, C.; Picerno, A.; Gallone, A.; Vulpi, M.; Vitarelli, A.; Ditonno, P.; Cascione, M.; Sallustio, F.; et al. Analysis of the Physico-Chemical, Mechanical and Biological Properties of Crosslinked Type-I Collagen from Horse Tendon: Towards the Development of Ideal Scaffolding Material for Urethral Regeneration. Materials 2021, 14, 7648. https://doi.org/10.3390/ma14247648
Gallo N, Natali ML, Curci C, Picerno A, Gallone A, Vulpi M, Vitarelli A, Ditonno P, Cascione M, Sallustio F, et al. Analysis of the Physico-Chemical, Mechanical and Biological Properties of Crosslinked Type-I Collagen from Horse Tendon: Towards the Development of Ideal Scaffolding Material for Urethral Regeneration. Materials. 2021; 14(24):7648. https://doi.org/10.3390/ma14247648
Chicago/Turabian StyleGallo, Nunzia, Maria Lucia Natali, Claudia Curci, Angela Picerno, Anna Gallone, Marco Vulpi, Antonio Vitarelli, Pasquale Ditonno, Mariafrancesca Cascione, Fabio Sallustio, and et al. 2021. "Analysis of the Physico-Chemical, Mechanical and Biological Properties of Crosslinked Type-I Collagen from Horse Tendon: Towards the Development of Ideal Scaffolding Material for Urethral Regeneration" Materials 14, no. 24: 7648. https://doi.org/10.3390/ma14247648